INTRODUCTION
Knowledge on the biodiversity of terrestrial invertebrates in the phytogeographic domain of the Caatinga biome (semiarid scrubland) in Brazil is far from satisfactory (Magalhães 2012), especially with regards to land snails. Some studies report the occurrence of these organisms in heterogeneous habitats, demonstrating broad adaptation to severe and adverse climatic conditions (Simone 2006, Simone & Casati 2013, Salvador & Simone 2014). To date, ants, bees, beetles and spiders are among the main invertebrates studied in this biome (Iannuzzi et al. 2003, Leal 2003, Maia et al. 2003, Zanella & Martins 2003, 2005, Carvalho & Brescovit 2005, Hernández 2005, Martins et al. 2005, Quinet & Tavares 2005, Leal et al. 2017).
The genus Gastrocopta Wollaston, 1878 includes land microsnails belonging to the family Gastrocoptidae Pilsbry, 1918, with dozens of recent and fossil species recorded throughout the Europe (Stworzewicz & Prysiazhniuk 2006, Salvador & Rasser 2016), Asia (Pokryszko & Stworzewicz 2003, Pokryszko et al. 2009, Prysiazhniuk 2015, Meng & Bößneck 2018), Africa (Herbert & Willows-Munro 2022), North America (Nekola & Coles 2001, Pierce & Constenius 2001), Central America (Herbert & Willows-Munro 2022), Caribbean (Chan & Lau 2020), South America (Simone 2006, Kotzian & Amaral 2013, Cunha et al. 2015, Salvador et al. 2017, Miquel & Brito 2019, Batistão et al. 2021, Brito & Miquel 2022, Miranda et al. 2023) and Oceania (Solem 1991, Pokryszko 1996, Whisson & Köhler 2012).
Such gastrocoptids live in terrestrial ecosystems in tropical (including semiarid regions) and temperate regions associated with leaf litter, fallen trunks, moss, shrubs, granite, limestone, clay, schist, sandy soils, flood debris, grassy habitats, cliffs and farming areas (Solem 1991, Nekola & Coles 2001, Pokryszko et al. 2009, Stanisic et al. 2010, 2018, Miquel & Brito 2019, Brito & Miquel 2022, Herbert & Willows-Munro 2022). Snails of this genus are usually calciphiles (Solem 1991, Vermeulen & Whitten 1998, Hotopp et al. 2013), but some species apparently do not demonstrate the typical affinity with calcium-rich habitats (Pokryszko et al. 2009, Herbert & Willows-Munro 2022).
Previous studies recorded Gastrocopta barbadensis (Pfeiffer, 1853) on Trindade Island in the state of Espírito Santo (southeastern Brazil) (Cunha et al. 2015) and listed G. iheringi (Suter, 1900), G. oblonga (Pfeiffer, 1852), G. servilis (Gould, 1843), G. solitaria (Smith, 1890) and G. pellucida hordeacella (Pilsbry, 1890), with records of occurrence in northeastern, southeastern or southern Brazil (Simone 2006). Among these, G. servilis is recognised as an introduced species with widespread distribution (Chan & Lau 2020, Herbert & Willows-Munro 2022). In Brazil, there is no mention of this taxon as an exotic species (Simone 2006, Salvador et al. 2021). More recently, Salvador et al. (2017) described G. sharae Salvador, Cavallari et Simone, 2017 from caves with water bodies and clay substrate in the Cerrado biome (savanna) in the state of Goiás (central Brazil).
Gastrocopta pellucida hordeacella is the most widely studied Gastrocopta species in Brazil. Individuals of this taxon have been recorded in a few states, such as Bahia (northeastern region), Minas Gerais, Rio de Janeiro and São Paulo (southeastern region) (Pinto-de-Oliveira & Almeida 1999, Simone 2006, Kotzian & Amaral 2013, Batistão et al. 2021, Miranda et al. 2023). Batistão et al. (2021) also found G. pellucida hordeacella associated with the pitcher plant Nepenthes graciliflora in southeastern Brazil and recognised this gastrocoptid as introduced.
This paper records the land microsnail Gastrocopta pellucida hordeacella for the state of Paraíba in an ecological park in the semiarid region of Brazil, expanding knowledge on the area of occurrence of the taxon in northeastern Brazil. The study area is subject to anthropogenic disturbances, especially those related to agricultural activities, increasing the likelihood of G. pellucida hordeacella as an anthropochorous subspecies occupying the region through the transport of cultivated plants.
MATERIAL AND METHODS
STUDY AREA
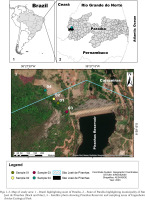
Engenheiro Ávidos Ecological Park is located in the municipalities of Cajazeiras and São José de Piranhas in the western extremity of the state of Paraíba in northeastern Brazil (Figs 1–5). The park is situated in a mountainous chain with shrub-herbaceous and arboreal vegetation (shrub-herbaceous stratum in all environments and closed tree formations at the highest points), hyperxerophilic phytophysiognomy (Araújo & Pereira 2016, Souto et al. 2019a) and various aquatic ecosystems (e.g., Piranha reservoir, streams and marginal lagoons) (Freitas 2012, Souto et al. 2019a, b). Average rainfall is 800 mm (rains concentrated between February and April) and temperature ranges from 23 to 33 °C (Feitosa 2000). Soil in the region is shallow sandy-clay at the base of the mountain and stony on the plateau, where rocky outcrops are found (Bandeira 2016). Engenheiro Ávidos is one of the priority areas for biodiversity conservation due to the unique biota, which has been studied very little over the years and is extremely susceptible to anthropogenic disturbances (Araújo & Pereira 2016, Feitosa et al. 2002, Souto et al. 2019a, b).
COLLECTION AND IDENTIFICATION
Collections of litter (leaf and soil) were carried out for one year (2017 to 2018) in a less impacted area of Engenheiro Ávidos Ecological Park. Collection areas included habitats bordering and entering the native vegetation. Substrates (10 to 15 kg of litter) from four different sampling areas were collected on 28.X.2017 and 11.XI.2017. The litter was left to dry in the sun and micromollusks were then screened under a stereomicroscope.
The material was processed at the Zoology Lab of the Centro de Formação de Professores [Professor Education Center] of Universidade Federal de Campina Grande [Federal University of Campina Grande]. Sampling was carried out with the authorisation of Sistema de Autorização e Informação em Biodiversidade (SISBIO 60982-1) [Authorization and Information System in Biodiversity], Instituto Chico Mendes de Conservação da Biodiversidade [Chico Mendes Biodiversity Conservation Institute], Ministério do Meio Ambiente [Ministry of the Environment].
Identification of the material was based on shell morphology (Batistão et al. 2021, Miranda et al. 2023) and performed under a stereomicroscope. Shells were also studied based on photographs taken with a stereoscopic microscope. The description of apertural barriers is based on Whisson & Köhler (2012: 17, fig. 1).
Specimens are deposited in the following scientific collections: UFPB MOL – Coleção de Invertebrados Paulo Young, Departamento de Sistemática e Ecologia, Universidade Federal da Paraíba, João Pessoa, Paraíba, Brazil.
RESULTS AND DISCUSSION
Gastrocoptidae Pilsbry, 1918
Gastrocoptinae Pilsbry, 1918
Gastrocopta Wollaston, 1878
Gastrocopta pellucida hordeacella (Pilsbry, 1890)
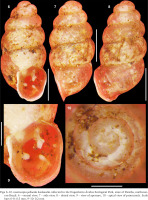
Figs 6–10
Gastrocopta pellucida hordeacella collected in the Engenheiro Ávidos Ecological Park, state of Paraíba, northeastern Brazil: 6 – ventral view, 7 – side view, 8 – dorsal view, 9 – view of aperture, 10 – apical view of protoconch. Scale bars 6–8: 0.5 mm, 9–10: 0.2 mm
Material examined. 1 shell (Figs 6–8) – UFPB.MOL-4071, Brazil (northeastern Brazil), state of Paraíba, municipality of São José de Piranhas, Engenheiro Ávidos Ecological Park, sampling area 2, 06°59′08″S, 38°27′24″W (380 m), litter, 28.10.2017, Evandro C. T. Abreu collector; 2 shells – UFPB.MOL-4072, Brazil (northeastern Brazil), state of Paraíba, municipality of São José de Piranhas, Engenheiro Ávidos Ecological Park, sampling area 1, 06°59′09″S, 38°27′23″W (376 m), litter, 28.10.2017, Evandro C. T. Abreu collector; 1 shell – UFPB.MOL-4073, Brazil (northeastern Brazil), state of Paraíba, municipality of São José de Piranhas, Engenheiro Ávidos Ecological Park, sampling area 3, 06°59′11″S, 38°27′21″W (371 m), litter, 28.10.2017, Silvio F. B. Lima collector; 1 shell – UFPB.MOL-4074, Brazil (northeastern Brazil), state of Paraíba, municipality of Cajazeiras, Engenheiro Ávidos Ecological Park, sampling area 4, 06°59′06″S, 38°27′26″W (380 m), litter, 11.11.2017, Evandro C. T. Abreu collector.
Characterisation (material examined). Shell orangish-brown to reddish-brown, thin, minute (about 1.6 mm in length), pupoid-oval. Apex obtuse, rounded. Protoconch smooth, bulbous, blunt, slightly nippled, with 1 whorl; transition to teleoconch abrupt, marked by a tenuous orthocline axial edge. Spire about 50% of total length, blunt-obtuse, rimate. Teleoconch with about 5 inflated, globose, convex whorls, which increase moderately in size, covered by closely spaced, prosocline, low growth striations. Body whorl inflated, oval: width and length about 45% and 53% of shell length, respectively. Suture well-marked, shallow, slightly channeled and oblique (diagonal) to columellar axis. Peristome oval, slightly reflexed (except on upper palatal region) and flattened in columellar area. Aperture oval, about 1/3 of total length, with 4 apertural barriers that do not obstruct the aperture; large, bifid parietoangular tooth (greater apertural barrier – upper portion thin, slightly curved to the right and lower portion large, bulbous) slightly displaced towards the upper corner of the parietal region, two palatal teeth (minute upper palatal tooth and larger lower palatal tooth, both well-spaced) and subhorizontal columellar tooth. Columellar and lower palatal tooth with similar vigor, contour and at certain distance from each other. Parietoangular tooth, lower palatal tooth and columellar tooth somewhat equidistant. Lips prosocline, thin, slightly reflexed. Suprapalatal region with distinct angulation.
Distribution. Northeastern Brazil – state of Paraíba: municipalities of Cajazeiras and São José de Piranhas in Engenheiro Ávidos Ecological Park (present study) and state of Bahia: Atlantic Forest region in the Contas River basin (Kotzian & Amaral 2013); southeastern Brazil – see Miranda et al. (2023).
Remarks. Gastrocopta pellucida hordeacella was originally studied in the United States, with no information on its natural habitat (Pilsbry 1890, Vanatta 1912). In Brazil, this gastrocoptid has been recorded in the northeastern and southeastern regions, with no information on distribution outside its natural limit (Pinto-de-Oliveira & Almeida 1999, Kotzian & Amaral 2013, Batistão et al. 2021). Gastrocopta pellucida hordeacella lives in the Atlantic Forest biome (Simone 2006, Kotzian & Amaral 2013) as well as ecological systems with rustic, deciduous and hyperxerophilous vegetation typical of the Caatinga phytogeographic domain (present study). All specimens studied here were collected from a dense layer of leaf litter accumulated at the base of a steep area with no incidence of calcium-rich habitats. This gastrocoptid has also been found in gardens of urban areas (Batistão et al. 2021, Miranda et al. 2023) associated with exotic ornamental vegetation (Batistão et al. 2021).
Engenheiro Ávidos Ecological Park suffers severe anthropogenic impact due to agricultural activities as well as the planting of invasive exotic vegetation. Neem (Azadirachta indica A. Juss., originally from India) is the main evergreen tree introduced in the study area, the seedlings of which are transported and cultivated throughout Brazil (Neves et al. 2013, Dantas et al. 2018). Shrub and tree seedlings widely distributed and cultivated by humans between regions, including within the boundaries of the ecological park, have immense potential with regards to the accidental transport of land snails introduced in Brazil, especially microsnails, such as Gastrocopta pelucida hordeacella. The congener G. servilis is a common anthropochorous gastrocoptid found mainly in anthropogenic tropical habitats (Herbert & Willows-Munro 2022).
The specimens studied have a shell somewhat smaller than 2 mm. The fully developed shells of Gastrocopta pellucida hordeacella analysed by Pilsbry (1890: 44), Batistão et al. (2021: fig. 2a–e) and Miranda et al. (2023: fig. 2A–B) also had a total length less than 2 mm. The individuals studied here and the one illustrated by Simone (2006: fig. 350) do not have the basal tooth. In contrast, the shells illustrated by Batistão et al. (2021: fig. 2a–c) and Miranda et al. (2023: fig. 2A–C) have a defined basal tooth, although it is not clearly seen in the aperture of some shells (Miranda et al. 2023: 113). The presence or absence of a basal tooth represents an intraspecific variation rather an incomplete developmental stage (e.g., juvenile or subadult) of the aperture of G. pellucida hordeacella. The protoconch of G. pellucida hordeacella in this study was smooth (see also Batistão et al. 2021: 2440, fig. 2f, Miranda et al. 2023: fig. 2D) with a clear transition to the teleoconch (present study), but the transition is not distinct in other studies (Batistão et al. 2021: 2440, fig. 2f, Miranda et al. 2023: fig. 2D).