INTRODUCTION
At the end of the 20th and beginning of the 21st century, it became noticeable that some land mollusc species were rapidly expanding their ranges in Eastern Europe (Gural-Sverlova et al. 2021, Adamova et al. 2022a, Balashov & Markova 2023b) and, in particular, in Ukraine (Gural-Sverlova et al. 2009, Gural-Sverlova & Gural 2017, 2020b, 2023a, Balashov & Markova 2023a, Gural-Sverlova & Rodych 2023, etc.) due to anthropochory and global climate change. These include the Caucasian snail Harmozica ravergiensis (Férussac, 1835) with already described findings in different regions of Ukraine (Gural-Sverlova & Timoshenko 2012, Balashov et al. 2018), in the south-east of Belarus (Ostrovsky 2022), and in Belgorod (Adamova et al. 2022b), Tver and Moscow (Schikov 2016) regions in the European part of Russia.
The discovery of H. ravergiensis in the vicinity of Ternopil in 2006 remained the most western known record of this species for almost two decades, and the only record known in Western Ukraine (Gural-Sverlova & Timoshenko 2012, Gural-Sverlova 2017). In the autumn of 2023, however, we found a large population of H. ravergiensis further west, in Lviv city. This new record deserves special attention, as the penetration of any introduced species of land molluscs into the largest settlement of Western Ukraine creates favourable conditions for its further spread in this area and can accelerate also its possible introduction into Central Europe, in particular, into Poland.
The observations in a popular citizen science database (iNaturalist 2024), illustrated with photographs, show that the current distribution of H. ravergiensis outside the Caucasus region is much wider than can be assumed from literature data (see above). Therefore, in addition to a detailed description of the new record of H. ravergiensis in Western Ukraine and a discussion of possible ways of its penetration into the studied area, we have analysed the general trends in the spread of H. ravergiensis outside its natural range. So far, no synthesis paper on this topic has been published. Despite being known in Eastern Europe since the 1990’s, it is not included in the guide to European non-marine molluscs by Welter-Schultes (2012).
MATERIAL AND METHODS
In Lviv, H. ravergiensis was found in large numbers along the periphery of the recently built residential complex Semytsvit (Figs 1–2) between Taras Shevchenko and Zolota Streets. The residential complex consists of 15 multi-storey buildings and more than two thousand apartments. The first building was commissioned at the end of 2016, and the last at the end of 2022. Snails were collected mainly along various fences (Figs 3–4), as well as on retaining walls stabilizing slopes (Fig. 2). In some places, H. ravergiensis was also recorded in small numbers among young ornamental plantings (Fig. 5). The area where H. ravergiensis was found was approximately 250 m long and up to 200 m wide. We inspected this area many times from mid-September to early November 2023.
Figs 1–5
H. ravergiensis in Lviv: 1, 2 – general view of a residential complex, 3–5 – different collection sites

Most of H. ravergiensis specimens were collected between the open parking lot near building No. 10 (Fig. 3, 49°50′47.3″N, 24°00′31.2″E) and buildings No. 17 (49°50′50.4″N, 24°00′33.6″E) and No. 16 (49°50′54.2″N, 24°00′28.6″E), as well as between buildings No. 2 (Fig. 5, 49°50′42.7″N, 24°00′42.5″E) and No. 18 (49°50′49.3″N, 24°00′41.0″E) and house No. 7 on Zolota Street (49°50′47.5″N, 24°00′46.0″E) bordering the Semytsvit residential complex. These two plots are designated as “Lviv-1” and “Lviv-2” in Table 1. For measurements, we used adults of H. ravergiensis from these sites, which had fully formed shells with the aperture margins distinct turned outward. The shells were measured with a calliper with an accuracy of 0.1 mm according to the standard method. H. ravergiensis was identified according to the monograph by Schileyko (1978).
To analyse the current distribution of H. ravergiensis outside the Caucasus region, we used the stock materials of the State Museum of Natural History of the National Academy of Sciences of Ukraine in Lviv (hereinafter referred to as SMNH NANU), partially described in a previous publication (Gural-Sverlova & Timoshenko 2012), literature data (Balashov et al. 2013, Schikov 2016, Balashov et al. 2018, Ostrovsky 2022, Adamova et al. 2022b, Balashov & Markova 2023a) and critically analysed observations from two citizen science databases (iNaturalist 2024, UkrBIN 2024). The latter were used only if photographs of live snails or empty shells allowed reliable identification of H. ravergiensis (Appendix 1). iNaturalist is a joint initiative by the California Academy of Sciences and the National Geographic Society. Currently, it brings together almost three million scientists and naturalists from around the world. UkrBIN (Ukrainian Biodiversity Information Network) is a web project and application documenting biodiversity, launched in cooperation with the Schmalhausen Institute of Zoology of the National Academy of Sciences of Ukraine (Kyiv). When mapping, all records within one settlement are shown as one point.
The shells of H. ravergiensis specimens of different ages collected in Lviv (100 in total) are stored in the malacological collection of SMNH NANU (inventory Nos. 5240–5242, 5255). There are also shells of this species from the Donetsk and Ternopil regions of Ukraine and Armenia, used for comparison. Most of them are mentioned in the latest catalogue of the museum’s collection of land molluscs (Gural-Sverlova & Gural 2020a). In addition, a sample of H. ravergiensis, collected in September 2023 in the village of Maidan (Kramatorsk district, Donetsk region, inventory No. 5440, coll. S. Pisaryev), was transferred to the museum last year.
Lviv is the largest city in the west of Ukraine, a major cultural, scientific, educational and industrial centre as well as an important transport hub. At the beginning of 2022, the population was about 717 thousand. Lviv is located in the western part of the Podolian Upland, on the main European watershed separating the river basins flowing into the Black and Baltic Seas, in the zone of deciduous forests. The climate is temperate continental, DFb according to the Köppen-Geiger classification. The average temperature is −2.7 °C in January and +19 °C in July. The average annual precipitation is 767 mm, most of which falls during the warm season. The natural soil cover is characterised by alternating gray and dark gray podzolised soils with podzolised chernozems. In addition, the city has a lot of bulk soil, often with large amounts of construction waste. Lviv has a complex terrain (elevation differences from 290 to 413 m above sea level) and a wide variety of landscapes. Back in the middle of the 19th century, more than half of the territory of the present city was occupied by beech forests (Kucheriavyi 1984), only fragments of which have survived today.
RESULTS
In the middle of September, living individuals of H. ravergiensis of different ages (Fig. 6) were present in the studied area of Lviv. We also found the following species of land molluscs there: Laciniaria plicata (Draparnaud, 1801), Arion distinctus Mabille, 1868, A. vulgaris Moquin-Tandon, 1855, Zonitoides nitidus (O. F. Müller, 1774), Oxychilus draparnaudi (Beck, 1837), O. translucidus (Mortillet, 1854), Limax maximus Linnaeus, 1758, Deroceras reticulatum (O. F. Müller, 1774), Krynickillus melanocephalus Kaleniczenko, 1851, Xerolenta obvia (Menke, 1828), Monacha cartusiana (O. F. Müller, 1774) (Fig. 7), M. fruticola (Krynicki, 1833) (Fig. 8), Helix pomatia Linnaeus, 1758, Cornu aspersum (O. F. Müller, 1774) (Fig. 9), Caucasotachea vindobonensis (C. Pfeiffer, 1828), and Cepaea hortensis (O. F. Müller, 1774) (Figs 10–11). For identification of A. distinctus, O. draparnaudi, D. reticulatum, X. obvia, M. cartusiana, M. fruticola, as well as C. hortensis individuals with atypical (dark) lip colour (Fig. 11), the structure of the genitalia was used.
Figs 6–11
The first sample of H. ravergiensis from Lviv (6) and other introduced Helicoidea species found in the same area (7–11): 7 – Monacha cartusiana, 8 – M. fruticola, 9 – Cornu aspersum, 10, 11 – Cepaea hortensis

Most of the species we found, except H. ravergiensis, M. fruticola and C. hortensis, were represented by a few individuals or even single empty shells, as C. aspersum and C. vindobonensis. The most abundant species was H. ravergiensis. In addition to the samples stored in the malacological collection of SMNH NANU (see Material and Methods), we observed at least several hundred more living specimens of this species in the studied area. In some places, joint aggregations of H. ravergiensis and M. fruticola were found, especially near buildings No. 2 and 17. A single shell of an adult C. aspersum (Fig. 9) and a small population of C. hortensis with dark lip in all pink and brown shells (Fig. 11) were found among young ornamental plantings near building No. 2, including low-growing coniferous and deciduous shrubs and young yews (Fig. 5). Specimens of C. hortensis, collected at this (Fig. 10, left) and other sites (Fig. 10, right), differed also in shell size and body colouration. In the rest of the residential complex, they had only a light body, a white lip, and a yellow or white shell without dark spiral bands.
The width (diameter) of the measured shells of adult specimens of H. ravergiensis from Lviv varied from 14.0 to 19.0 mm (on average, about 16 mm), and their height – from 10.2 to 14.7 mm (on average, about 12 mm). For more detailed measurement results and their comparison with data from other localities in Ukraine, see Table 1. In both Lviv samples, the shells were statistically significantly larger than in other samples of H. ravergiensis from Ukraine that we measured.
The shells had a moderately compressed shape and a narrow umbilicus, partially covered by the columellar edge of the aperture turned outward (Fig. 12). Numerous small granules were clearly visible in places on the shell surface (Fig. 13). In places, this surface could be described rather as crumpled than granular, without regular sculpture. In some places, there were also coarse light radial wrinkles, better visible under the suture (Fig. 13). The shell colour varied from whitish to light horny (Fig. 12). A white spiral band, poorly visible on light-coloured shells (Fig. 12, left), runs along the periphery of the whorls. In horn-coloured shells, the area under the suture was usually also lighter in colour (Fig. 12, centre). Occasionally the horny colour reached the very suture (Fig. 12, right).
Figs 12–13
Shells of H. ravergiensis from Lviv: 12 – three adult specimens with different colouration, 13 – surface sculpture. Scale bars 1 cm (12), 1 mm (13)

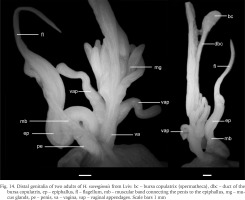
The genitalia of dissected specimens from Lviv (Fig. 14) were typical of H. ravergiensis (Schileyko 1978, 2005). The penis is short. The epiphallus is long, curved, connected to the penis by a massive muscular band. The flagellum is also long. There are two vaginal appendages, the size and shape of which differ little from the mucous glands.
Fig. 14
Distal genitalia of two adults of H. ravergiensis from Lviv: bc – bursa copulatrix (spermatheca), dbc – duct of the bursa copulatrix, ep – epiphallus, fl – flagellum, mb – muscular band connecting the penis to the epiphallus, mg – mucus glands, pe – penis, va – vagina, vap – vaginal appendages. Scale bars 1 mm
Analysed data from various sources (see Material and Methods) shows that H. ravergiensis has spread quite widely beyond the Caucasus region (Fig. 15). This species has now been reliably recorded in 12 administrative regions of Russia, 7 administrative regions of Ukraine, as well as in Gomel (Belarus) and Bishkek (Kyrgyzstan) (Table 2). Its known range reaches the Tver region of Russia in the north, the Southern Ural in the northeast, Central Asia in the southeast, and Lviv (Western Ukraine) in the west (Fig. 15).
Fig. 15
Reliable records of H. ravergiensis outside the Caucasus with Ciscaucasia: blue – personally examined material mainly stored at SMNH NANU, red – literature data, brown – observations from databases
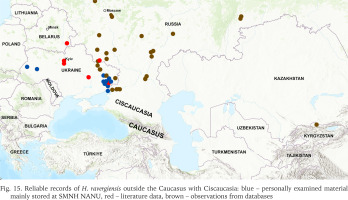
In Ukraine, H. ravergiensis is distributed mainly in the east of the country, especially in the Donetsk region, where it has been known since 1990 (Fig. 16). Recently it was also discovered in the administrative centres of Luhansk and Kharkiv regions (Table 2). A few localities of H. ravergiensis are also known in Central (Kyiv and Dnipropetrovsk regions) and Western (Lviv and Ternopil regions) Ukraine. The first records in these parts of the country were made in 2006 (Fig. 17) and 2015 (Table 2), respectively. In the southern part of Ukraine, from the Odesa region in the west to the Zaporizhzhia region in the east, H. ravergiensis has not yet been found. One specimen photographed in Simferopol (Crimea) in 2021 and identified as H. ravergiensis in the database iNaturalist (observation 102401006) in our opinion may well be Monacha fruticola, native and widespread in Crimea.
DISCUSSION
SPECIFICS OF THE LVIV POPULATION OF H. RAVERGIENSIS AND THE LAND MOLLUSC COMPOSITION IN THIS AREA
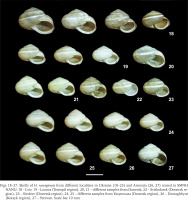
Lviv specimens of H. ravergiensis differ from other samples of this species from Ukraine, measured by us personally (Gural-Sverlova & Timoshenko 2012) or described in the literature (Balashov et al. 2018), on average by a larger shell size. This is clearly shown not only by the results of a biometric study (Table 1), but also by a comparison of different-sized shells of adult H. ravergiensis from Lviv (Fig. 18) with those from other localities in Ukraine (Figs 19–25) and even from the natural range of the species (Armenia, Figs 26–27) stored in SMNH NANU. According to Schileyko (1978, 2005), in the Caucasus, the shell width in H. ravergiensis varies from 12 to 18 mm, while in the measured shells from Lviv it ranges from 14 to 19 mm (Table 1). According to Snegin & Adamova (2016), the average shell width in 7 measured samples from the city of Belgorod (southwest of European Russia) varied from 12.43 to 14.24 mm, and in a sample from the vicinity of Lake Sevan (Armenia) it was 13.05 mm. This is well consistent with data from Ukrainian populations of H. ravergiensis, except Lviv (Table 1). In other respects, Lviv specimens of H. ravergiensis did not differ from the available descriptions and images of this species from the Caucasus (Akramovsky 1976, Schileyko 1978, 2005) or other introduced Eastern European populations (Gural-Sverlova & Timoshenko 2012, Balashov et al. 2018, Ostrovsky 2022).
Figs 18–27
Shells of H. ravergiensis from different localities in Ukraine (18–25) and Armenia (26, 27) stored in SMNH NANU: 18 – Lviv, 19 – Lozova (Ternopil region), 20, 21 – different samples from Donetsk, 22 – Svitlodarsk (Donetsk region), 23 – Siedove (Donetsk region), 24, 25 – different samples from Yasynuvata (Donetsk region), 26 – Dzoraghbyur (Kotayk region), 27 – Yerevan. Scale bar 10 mm
Among 16 species of land molluscs collected in the same area of Lviv as H. ravergiensis and listed in Results, only a few can be considered native to Western Ukraine or at least for the western part of the Podolian Upland (Gural-Sverlova et al. 2022): L. plicata, Z. nitidus, X. obvia, C. vindobonensis, H. pomatia. The exact boundaries of the natural range of D. reticulatum in Europe are not known (Wiktor 2000: 509), but in Lviv and the surrounding area it is a typical synanthrope. Other species appeared in Western Ukraine only due to anthropochory, in many cases not earlier than the end of the 20th or beginning of the 21st century. The latter applies to A. vulgaris, K. melanocephalus, O. translucidus, M. cartusiana, M. fruticola, C. aspersum (Gural-Sverlova & Gural 2021a: table 2). This is the third known locality of M. fruticola in Western Ukraine. The previous two were discovered in 2018–2019 in and near Lviv (Gural-Sverlova & Gural 2020b).
Of interest is the unusual phenotypic composition of Cepaea hortensis near one of the buildings of the residential complex. In addition to pink shells with a dark lip, which have been recorded at some sites of Lviv and its immediate surroundings since 2019 (Gural-Sverlova & Gural 2021b, 2022a), there were also dark-lipped brown shells (one at the top left in Fig. 10, two at the top in Fig. 11), first discovered in Ukraine. We found that C. hortensis with an atypically coloured lip spreads from at least two large garden centres in the Lviv region – in Pidbirtsi (Gural-Sverlova & Gural 2022a) and Horodok (Gural-Sverlova & Gural 2023b). In the latter case, several specimens of C. aspersum were also found along the garden centre fence. However, brown shells have not yet been recorded in C. hortensis from Horodok. The only three brown shells of C. hortensis found in 2021 near the garden centre in Pidbirtsi had a light lip, typical for this species, a less intense brown colour (from yellowish brown to light brown) and a larger size compared to the dark-lipped pink shells (Gural-Sverlova & Gural 2022a: >fig. 5). The light body and limited variability in the shell colouration recorded at some other sites of the residential complex (three snails on the right in Fig. 10) are characteristic of the descendants of the primary introduction of C. hortensis into Western Ukraine (Gural-Sverlova & Gural 2021b, 2022a), which occurred no later than the second half of the 20th century (Gural-Sverlova & Gural 2021b). We observed such specimens in the area of Zolota Street back in the late 1990s.
POSSIBLE WAYS OF INTRODUCTION OF H. RAVERGIENSIS INTO LVIV
Since the introduction of land molluscs often occurs unintentionally, for example, with plant seedlings, transported cargo, vehicles, etc., only in a few cases it is possible to establish the exact origin of introduced populations. The same applies to the mechanisms of penetration of some species into one or another territory. Plant nurseries and garden centres play an important role in the spread of alien species of land molluscs (Bergey et al. 2014). In recent decades, this has been clearly confirmed, for example, by the rapid spread of Cepaea nemoralis (Linnaeus, 1758) in the urbanised areas of Eastern Europe (Gural-Sverlova et al. 2021), in particular in Ukraine (Gural-Sverlova et al. 2024). However, in recent years we have deliberately examined the surroundings of about a dozen garden centres in Lviv and the Lviv region, operating or recently closed, where we often found Cepaea and some other alien mollusc species, but never H. ravergiensis or M. fruticola (see below).
We found clear evidence of the introduction of some mollusc species together with seedlings of ornamental plants at one site of the studied residential complex in Lviv (Fig. 5), where atypically coloured specimens of C. hortensis (see above) and one empty shell of C. aspersum were collected. However, under the same young ornamental bushes we found only single individuals of H. ravergiensis, which could migrate there later from nearby places of mass accumulation of this species. Unfortunately, we were unable to obtain any information about the sources of landscaping for this residential complex.
The first record of H. ravergiensis in Western Ukraine was made near a quarry in the vicinity of Ternopil (Gural-Sverlova & Timoshenko 2012), and the second was made along the periphery of a recently built residential complex in Lviv. Therefore, one version of the possible spread of this species was its accidental transportation with construction materials, on trucks transporting them or other construction equipment. To test this hypothesis, we examined the surroundings of several new buildings (commissioned or with construction not yet completed) of the same developer (construction company Interhal-Bud), as well as several other new residential complexes along Taras Shevchenko Street. We did not find either H. ravergiensis or M. fruticola anywhere. Along the fences of two residential complexes under construction, we found only a single species of relatively large snails (X. obvia or M. cartusiana), which can attach to vehicles (Trautner 2000, Kurek & Najberek 2009, Gural-Sverlova et al. 2022, Gural-Sverlova & Gural 2023a).
There is no evidence that H. ravergiensis entered the study area via transport. However, we can assume that cars can play an important role in its further spread throughout Lviv, and then also to other settlements of the Lviv region. The residential complex has one underground parking. However, most of the residents’ personal cars are parked near the buildings (Figs 1–2), often next to the fences on which we collected H. ravergiensis (Fig. 3). In rainy weather, snails actively climb fences and supporting walls that strengthen slopes. During subsequent dry periods, some of them remain attached to these surfaces, maintaining viability. At least several individuals of H. ravergiensis may likely use cars parked nearby in a similar manner. Cases of transporting snails attached to vehicles have been described for some other species of the families Hygromiidae and Geomitridae (Trautner 2000, Aubry et al. 2006, Kurek & Najberek 2009). This may be the reason for the rapid spread of M. cartusiana in Lviv (Gural-Sverlova & Gural 2023a) and, in general, in Western Ukraine (Gural-Sverlova & Gural 2022b). Unfortunately, we could not examine directly the cars in the protected area of the residential complex.
DIFFERENT PATTERNS OF THE PRESENT DISTRIBUTION OF H. RAVERGIENSIS AND M. FRUTICOLA IN UKRAINE
In the studied area of Lviv, the Caucasian species H. ravergiensis and the Crimean M. fruticola often live together. Although, in general, H. ravergiensis is somewhat more widespread there and is more numerous than M. fruticola. Such cohabitation of two alien species, very rarely found in Western Ukraine, suggests their possible joint introduction. In Lviv, and less often in its environs, we have repeatedly recorded the joint introduction of two Cepaea species (Gural-Sverlova et al. 2024) spreading from garden centres. We also know of a similar case of the joint introduction of C. nemoralis and C. aspersum into the household plot of one mansion in Lviv (personal unpublished data). Two cryptic species, Monacha claustralis (Rossmässler, 1834) and M. cartusiana, sometimes living together in Lviv and the surrounding area (Gural-Sverlova & Gural 2022b, 2023a), can probably also spread together, but attached to cars, and not from gardens centres.
However, H. ravergiensis and M. fruticola have not only different origins but also very different patterns of their present distribution in Ukraine. So far, only three cases are known when these two species were found in the same settlement (Fig. 28) – in the cities of Luhansk, Kyiv and Lviv. Moreover, their cohabitation in the same area was recorded only in Lviv. Previously, we observed a similar case of cohabitation of H. ravergiensis and M. fruticola only outside Ukraine, in Armenia, where an introduced population of M. fruticola was found in summer cottages near Yerevan (Dzoraghbyur, Kotayk region) (Gural-Sverlova et al. 2017).
Fig. 28
Records of H. ravergiensis and M. fruticola in Ukraine outside Crimea: red – only M. fruticola, brown – only H. ravergiensis, blue – both species were found in the same settlement (Lviv, Kyiv, Luhansk). In Crimea, M. fruticola is a native and widespread species, while H. ravergiensis has not yet been reliably recorded
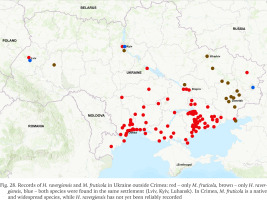
In addition to Crimea, M. fruticola is now widespread in the western and central parts of the steppe zone of Ukraine (Gural-Sverlova 2018), occasionally occurring in the eastern, central and western parts of the country (Fig. 28). In contrary, most of the known records of H. ravergiensis were made in the east of Ukraine, namely in the Donetsk region (Fig. 28, Table 2). Even in the neighbouring Zaporizhzhia region, despite recent detailed studies of land molluscs in urbanised and suburbanised areas (Gural-Sverlova et al. 2018, Gensytskyi 2021), H. ravergiensis has not been found, while M. fruticola is a background species.
CAUCASIAN MOLLUSCS THAT HAVE RAPIDLY EXPANDED THEIR RANGES IN THE SECOND HALF OF THE 20TH AND EARLY 21ST CENTURIES
H. ravergiensis is not the only land mollusc species of Caucasian origin that significantly expanded its range at the end of the 20th and beginning of the 21st centuries. A similar, and even stronger, trend was noted for two slug species, K. melanocephalus and Deroceras caucasicum (Simroth, 1901). Almost until the very end of the 20th century, K. melanocephalus was mentioned in Ukraine only for the mountainous Crimea; the earliest known collection material from there dates back to 1916 (Likharev & Wiktor 1980). This species was first recorded in Kyiv in 1998 (Korol & Korniushin 2002), and in Lviv in 2000 (Gural-Sverlova & Gural 2021a), soon becoming common in both cities. Moreover, K. melanocephalus even began to actively invade forest biotopes around Lviv, which was previously unusual for introduced species of land molluscs in this area (Gural-Sverlova 2017). Later, K. melanocephalus was recorded in other localities of the western, central and eastern administrative regions of Ukraine (Table 3).
In neighbouring Belarus, K. melanocephalus was first discovered in 2009 in Minsk (Ostrovsky 2017), and over the next ten years it was recorded in different parts of the country, and even in the Berezinsky Biosphere Reserve (Zemoglyadchuk 2020). In the centre of the East European (Russian) Plain (European Russia), K. melanocephalus has also been known since 2009 (Schikov 2016). Already in the 1990s, K. melanocephalus entered Northern (Latvia) and Central (Germany) Europe (Meng & Bößneck 1999, šteffek et al. 2008), where to date it has also been recorded in some other countries (Stalažs et al. 2018, von Proschwitz 2020, Turóci et al. 2020, Čejka et al. 2021, Maćkiewicz & Borys 2023, Watz & von Proschwitz 2023).
Equally impressive is the expansion of the range of D. caucasicum. Already in the middle and second half of the 20th century, this species spread to the countries of Central Asia (Prozorova & Fomenko 2015, Schikov 2017). In the 1990s, D. caucasicum was first discovered in the Russian Far East, where it has not only become a dangerous pest but can also threaten natural ecosystems (Prozorova & Fomenko 2015). In Ukraine, from the second half of the 20th century (Likharev & Wiktor 1980) until the beginning of the 21st century, D. caucasicum was mentioned only for Crimea. Then it began to be found more and more often in different parts of Ukraine (Gural-Sverlova et al. 2009, Gural-Sverlova 2017). More detailed information about its present distribution in Ukraine can be found in Table 3. D. caucasicum has been known for the centre of the East European (Russian) Plain (European Russia) since 2004 (Schikov 2016), and for Belarus since 2016 (Ostrovsky 2018). In Central or Northern Europe, unlike K. melanocephalus mentioned above, D. caucasicum has not yet been recorded.
Another slug of Caucasian origin, Boettgerilla pallens Simroth, 1912, spread throughout Europe even earlier. It was recorded in many European countries from the middle (the first known find in 1949 in Germany) to the end of the 20th century (Reise et al. 2000: table 1). In the second half of the 20th century, introduced populations of B. pallens were also discovered in Central Asia (Likharev & Wiktor 1980, Schikov 2017) and North America (Reise et al. 2000). It has been known in the European part of Russia since 1950 (Schikov 2016). In Ukraine, B. pallens was found in Crimea (Balashov & Baidashnikov 2012), Lviv, Ivano-Frankivsk, Chernivtsi (Gural-Sverlova & Gural 2021a), Kyiv, Vinnytsia, Poltava, Luhansk (Balashov 2016) and Dnipropetrovsk regions (iNaturalist 2024).
Among the land molluscs that noticeably expanded their ranges in Ukraine due to anthropochory, Oxychilus translucidus (Mortillet, 1854) and Limacus maculatus (Kaleniczenko, 1851) were also classified as species of Caucasian or Caucasian-Crimean origin (Balashov et al. 2018). However, the boundaries of their natural ranges are unclear (see below), so these species might be more correctly called Pontic rather than Caucasian. For this reason, we did not include either O. translucidus or L. maculatus in the review of Caucasian land molluscs recorded in Ukraine (Gural-Sverlova 2017).
O. translucidus has a very strong tendency to synanthropisation, which makes it difficult to determine its exact origin (Riedel 1966, 1989). Even in the Caucasus, it was found almost exclusively in anthropogenic habitats, occasionally in natural biotopes located next to them (Riedel 1966). In the 19th century, O. translucidus was described first from the Black Sea coast of Asia Minor (Trabzon, Türkiye), and then as Hyalinia komarowi O. Boettger, 1881 from the Western Caucasus. Welter-Schultes (2012) suggests that O. translucidus may have originally occurred in Eastern Türkiye and Northern Iran, and was later introduced into some European countries as well as Israel. Riedel (1964) assumed that O. translucidus may be native even to the Balkan Peninsula (Bulgaria).
In Ukraine, O. translucidus was first mentioned by Baidashnikov (1992) for the Kyiv region; now it is known from different parts of the country (Balashov & Gural-Sverlova 2014, Gural-Sverlova & Obednina 2018: fig. 1). The spread of this species over Ukraine could have occurred from different sources. For example, it could have reached at least the west of the country from Central Europe. For comparison, O. translucidus was first found in Poland (greenhouses of the botanical garden in Warsaw) in 1929, and in Western Ukraine 80 years later (Gural-Sverlova & Gural 2021a: table 2). Since 2019, O. translucidus has been known from Lviv, occasionally occurring in places where ornamental plants were recently planted. There is one known record on the fence of a recently closed garden centre.
L. maculatus is probably originally occurred in the Black Sea area, i.e. the Caucasus, Crimea and the Black Sea Coasts of Romania and Bulgaria (Wiktor 2001) and possibly Anatolia (Wiktor & Norris 1982). In addition to the Crimea, part of the natural range of this species in Ukraine may be also the Donetsk Upland in the east of the country (Balashov 2016, Balashov & Markova 2021), which was discussed in more detail in Gural-Sverlova & Rodych (2023).
In contrast to H. ravergiensis (Fig. 28, Table 3), D. caucasicum and K. melanocephalus (Table 3), L. maculatus was reliably recorded at the end of the 20th and beginning of the 21st centuries throughout the Southern Ukraine outside Crimea – in the Odesa, Mykolaiv, Kherson and Zaporizhzhia regions (Gural-Sverlova & Rodych 2023: fig. 3). In general, the present distribution of L. maculatus in Ukraine is more similar to that of Brephulopsis cylindrica (Menke, 1828) (Vychalkovskaya 2008: fig. 1), the above-mentioned M. fruticola (Fig. 28) or Xeropicta derbentina (Krynicki, 1836) (Gural-Sverlova & Gural 2017: fig. 2b). For the first two species, the initial source of dispersal was the Crimea. X. derbentina could theoretically spread both from the Crimea and from the Crimea and the Caucasus simultaneously.
Unlike the Caucasian slug species described above, the known distribution area of H. ravergiensis outside the Caucasus remains limited mainly to Eastern Europe, with a few records in the Southern Ural and Central Asia (Fig. 15, Table 2). Although the potential spread of this species throughout Lviv and the Lviv region, in our opinion, can significantly increase the likelihood of its future penetration into Poland. Outside the Caucasus, H. ravergiensis is usually recorded in settlements, less often in their immediate vicinity. Many known records of H. ravergiensis were made in cities that are the centres of administrative regions (Table 2). Nevertheless, in the Donetsk region of Ukraine, not all of the habitats found outside settlements were of a clearly anthropogenic nature (Gural-Sverlova 2017). We cannot yet say whether H. ravergiensis tends to naturalise and penetrate natural biotopes, like K. melanocephalus or D. caucasicum.
In its natural range, H. ravergiensis inhabits open or partially shaded biotopes: sparse forests, bushes, forest edges and glades (Akramovsky 1976, Schileyko 1978). Therefore, many anthropogenic areas, in particular in settlements, may be suitable for it. In the Caucasus region (Armenia), H. ravergiensis was also repeatedly found in anthropogenic habitats: gardens (Akramovsky 1976), summer cottages (Gural-Sverlova et al. 2017), as well as in the botanic garden of Yerevan (Gural-Sverlova et al. 2017).