Publication LSID: urn:lsid:zoobank.org:pub:657D4852-7200-4523-AB79-3277F694E655
INTRODUCTION
The finned octopods (suborder Cirrata) are typically deep-dwelling octopods that differ from more “normal” typically shallower-dwelling incirrate octopods (suborder Incirrata) in possessing a robust internal shell, fins, and cirri that flank each row of suckers along the arms (Collins & Villanueva 2006). However, despite the growing number of recognised taxa, 52 species in Sept 2024 (World Register of Marine Species (WoRMS)), and increasing work on this group in recent years, the taxonomy and alpha diversity of these octopods remains imperfectly known. This is especially true for waters surrounding Australia, and more generally through the southwestern Pacific and Indian Oceans, which have been inadequately sampled using appropriate collection equipment and strategies for cirrates, and from where various unrelated and historically important species have been inconsistently documented (Hoyle 1885, Thiele 1915, Massy 1916, Berry 1918, Nesis 1987). With intense scrutiny of existing collections over the last ~25 years the cirrates in this broad region have received increased taxonomic attention (O’Shea 1999, O’Shea & Lu 2002, Verhoeff & O’Shea 2022, Verhoeff 2022, 2023a, 2024) – a cumulative effort that has resulted in considerable improvement in knowledge of the cirrate fauna of this region, from where three described cirrate taxa were reported from Australian waters in 2020, including Antarctic/sub-Antarctic territories, but 11 are now documented (to 2024). However, as the more distinctive or common representatives in these collections are described, it becomes increasingly apparent that both further species await description and that the contentious higher classification of these octopods has yet to be fully resolved.
Two problematic, poorly represented and highly gelatinous genera in collections from these waters, are Cirroteuthis Eschricht, 1836 and Cirrothauma Chun, 1911. These two genera have routinely been attributed to the family Cirroteuthidae Keferstein, 1866, for which diagnostic features include a secondary web connecting a large primary web to each arm, elongate threadlike pairs of cirri between suckers, and “sepioid” gills (Verhoeff 2023b, MolluscaBase 2024). Cirroteuthis (type species C. muelleri Eschricht, 1836) has a large, dorsally positioned “saddle-shaped” shell, modified suckers in the central portion of each arm (with vestigial sucker chambers and long stalks), and a prominent web nodule at the junction of the web and ventro-lateral face of each arm (Voss & Pearcy 1990, Bizikov 2004, Collins & Villanueva 2006, Verhoeff 2022). Cirrothauma (the type species C. murrayi Chun, 1911, and others mentioned shortly) has proportionally much longer arms, lacks web nodules, and its shell is “butterfly shaped” (Aldred et al. 1983). Despite similarities in what are considered diagnostic traits of the family, these two genera do not look particularly similar; they differ most notably in the exaggerated arm length and larger size of the latter.
Species of Cirroteuthis are mostly meso- to abyssopelagic, with video imagery revealing them to swim with their fins or to passively drift well-off the seafloor with webs and arms outstretched (Pearcy & Beal 1973, Jamieson & Vecchione 2022, Golikov et al. 2023); they may also briefly contact the seabed possibly to feed (Golikov et al. 2022). Sporadic records of Cirroteuthis from this region have been made from off New Zealand (O’Shea 1999), southeastern Australia (Verhoeff 2022), and southwestern Australia (Naturaliste Plateau) (Nesis 1987).
The exclusively meso- to abyssopelagic Cirrothauma (encompassing putative C. murrayi and C. magna) has been reported sporadically from around Australia and the adjacent regions of the Indian Ocean and southern Pacific (Atlantis Bank, southwestern Indian Ocean (Lindsay et al. 2000); off Heard Island, southern Indian Ocean (Verhoeff 2023a); New Zealand (O’Shea 1999); New Caledonia (Roux 1994); and the northern and southeastern Pacific (Aldred et al. 1983)). Cirrothauma murrayi Chun, 1911, the type species of this genus, has vestigial eyes that lack lenses and are embedded below the skin, and modified suckers (that lack an acetabulum chamber and are atop elongate stalks) (Aldred et al. 1983). A second species, described as Cirroteuthis magna Hoyle, 1885, was placed in this genus by O’Shea (1999) and Verhoeff (2023a), but while it is more Cirrothauma-like in shell morphology and arm form, it has normal and well-developed eyes and relatively normal and functional suckers (with acetabula and minimal/absent stalks). These species, like Cirroteuthis, have been observed to drift well-above the seafloor with outstretched arms, or to swim using their fins (Roper & Brundage 1972, Villanueva et al. 1997, Guerrero-Kommritz et al. 2018).
While differences in characters and their states described for the few Cirroteuthis specimens reported from these more southern waters have differed from Arctic Cirroteuthis muelleri Eschricht, 1836, and these southern specimens have been attributed to “n. sp. A” (Nesis 1987) or “aff. muelleri” (Verhoeff 2022), sufficient and appropriately preserved, quality specimens have never been available for detailed morphological (or molecular) analysis. While this remains partially the case, additional material has been located that supports initial contentions that these specimens differ (O’Shea 1999) that compels us to describe them as new to facilitate a larger review of these and related taxa. By doing so we also resolve the systematic status of southeastern Pacific “Cirroteuthis” hoylei Robson, 1932, which we refer to a new genus along with C. magna. While limited, molecular data both supports the erection of this new genus and indicates the existence of further taxa.
MATERIALS AND METHODS
SPECIMEN COLLECTION AND ANALYSIS
Cirroteuthis reported on herein were collected on research surveys from 1992–2022. The oldest material was collected in 1992 by RV “Tangaroa” TAN9202 (Feb–Mar 1992, a pre-recruitment “orange roughy” survey on the north-east slope of Chatham Rise, New Zealand); excepting the very last tow at 2,000 m depth (in which the Cirroteuthis specimen was collected) to test the working range of a net monitor, all other TAN9202 trawls were made between 700 and 1,000 m depth (Hart et al. 1992 – unpublished cruise report), strongly suggesting that Cirroteuthis from New Zealand waters occurs at considerably greater depth than is currently trawled for commercial fishes. Additional material was collected during the Halieutique Profond (HALIPRO) 2 voyage in Nov 1996 by RV “Tangaroa” – a joint research voyage between France and New Zealand, which surveyed the Norfolk Ridges and southern areas of Loyalty Ridge close to New Caledonia (Grandperrin et al. 1997). More recent material was collected by the Australian Marine National Facility RV “Investigator”, under the Australian Commonwealth Scientific and Industrial Research Organisation (CSIRO), during mid-2017 CSIRO voyage “Sampling the abyss” (IN2017_V03, May–Jun 2017) surveying the benthic fauna of the eastern Australian lower slope and abyssal regions (CSIRO 2023a), and a late-2022 voyage “Valuing Australia’s Gascoyne Marine Park” (IN2022_V09, Nov–Dec 2022), assessing the biodiversity of a recently established Gascoyne Marine Park off northwestern Australia, including lower slope and abyssal regions (CSIRO 2023b). Specimens were collected by orange roughy wing trawl, 16 mm cod-end (HALIPRO 2); demersal fish trawl (TAN9202); beam trawl (IN2017_V03); and by both beam trawl and scampi-style demersal trawl (similar to a McKenna net but with a 30 m otter board spread vs. 90 m in McKenna-type nets) (IN2022_V02). Specimens were fixed in 5% formalin before being stored in 70–75% ethanol; one specimen (TAN9202) was frozen after capture and formalin-fixed after thawing.
Material from Australian museums, the Tasmanian Museum & Art Gallery (TMAG), Collections Facility, Rosny Park; Western Australian Museum (WAM), Perth; and Museums Victoria (MV), Melbourne was examined by TJV. Material from the Muséum National d’Histoire Naturelle (MNHN), Paris, and National Museum of New Zealand Te Papa Tongarewa (NMNZ), Wellington was examined by SOS. Detailed photography and data were provided for material at the Natural History Museum (NHM), London (former British Museum Natural History, BMNH), NMNZ, and the Smithsonian National Museum of Natural History (NMNH; former US National Museum, collection acronym USNM).
For production of some images a stacking camera macro-imaging setup was used (100 mm or 65 mm lens) with Passport linear actuator software (Visionary Digital), and initial image processing with Capture One imaging software (Phase One). Image stacking used either Zerene Stacker (Zerene Systems) or Picolay (Heribert Cypionka).
Measurements, counts, and indices follow Verhoeff & O’Shea (2022), which used revised and new indices for cirrate octopods. Beak measurements follow Verhoeff & O’Shea (2022) and O’Shea (1999). Commonly used abbreviations, indices, and symbols include: ⌀ – diameter, AGC – accessory gland complex, AL – arm length, ALI – arm length index (as % of TL), ASC – arm sucker count, CiL – cirrus length, CLI – cirrus length index (% of ML), CO1 – cytochrome c oxidase subunit 1, EDI – eye diameter index (% of ML), FL – fin length, FLI – fin length index (% of ML), FuL – funnel length, FuLI – funnel length index (% of ML), FW – fin width, FWI – fin width index (% of fin length), GLC – gill lamellae count, HW – head width, HWI – head width index (% of ML), ML – dorsal mantle length, MW – mantle width, MWI – mantle width index (% of ML), NA – not available, PA – pallial aperture gape, PAI – pallial aperture index (% of ML), SC – spermatophoric complex, SDI – sucker diameter index (% of ML), SHI – (shell) saddle height index (% of total shell length/height in antero-posterior axis), SSI – saddle span index (% of shell span), Stn – station, SuD – sucker diameter, SWI – saddle width index (% of shell width/thickness in dorso-ventral axis), TL – total length, WD – web depth. Beak indices: H%L – beak height as % of beak length, H%W – beak height as % of beak width, HL%L – hood length as % of beak length, WL%L – wing length as % of beak length. To prevent taxonomic ambiguity, the genera Cirrothauma and Cirroteuthis are spelled out in full at each mention (as opposed to citing Cirroth. and Cirrote.). The abbreviation ML refers to ‘mantle length’ generally, though for molecular analysis sections ML refers to ‘maximum likelihood’.
REMOTELY OPERATED VEHICLE OBSERVATIONS OF CIRROTEUTHIS
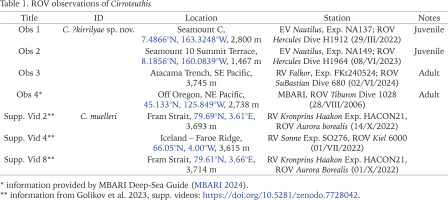
Remotely operated vehicle (ROV) observations of cirrate octopods from cruises by the EV “Nautilus” (Ocean Exploration Cooperative Institute), RV “Falkor” (Schmidt Ocean Institute) and Monterey Bay Aquarium Research Institute (MBARI) were investigated for putative Cirroteuthis observations across the Pacific (possibly attributable to Cirroteuthis kirrilyae sp. nov.). Four observations were identified from the central, northeastern and southeastern Pacific (Table 1). Images were extracted from high resolution video files; 10 cm scaling lasers were visible on some videos, but precise size calculation was not possible. For comparison, three Arctic Cirroteuthis muelleri ROV observations were also examined (Golikov et al. 2023, supplementary videos; see acknowledgements) (Table 1). Further live-animal images were sourced from the Norwegian Institute for Water Research (NIVA), and German Centre for Marine Biodiversity Research (DZMB).
PHYLOGENETIC ANALYSIS
Phylogenetic analysis was conducted using known specimens from family Cirroteuthidae available on GenBank (www.ncbi.nlm.nih.gov/genbank/) and BOLD (Barcode of Life System: https://www.boldsystems.org/) (Ratnasingham & Hebert 2007, Clark et al. 2016). Sequencing was also generously provided to the authors by Yves Cherel (Directeur de recherche au CNRS; sequencing by Louise Allcock, NUI Galway) for a “Cirrothauma” magna collected off Kerguelen Island, close to the type locality for the species; and Kathrin Bolstad (Associate Professor, Auckland University of Technology) provided CO1 sequencing for two Cirroteuthis kirrilyae sp. nov. collected off northwestern Australia (WAM S116614 and S116678). Sequences used are listed in Table 2.
Table 2
Available CO1 gene sequences for members of Cirroteuthoidea, with species identifications, localities and GenBank or BOLD accession numbers
Phylogenetic analysis was conducted in MEGA X (Kumar et al. 2018), sequence alignment using the MUSCLE method with settings for protein coding DNA (set to use “invertebrate mitochondrial”), and additional manual alignment checking to ensure no internal stop codons. Model selection (substitution model and rates among sites) for Maximum Likelihood (ML) phylogenetic tree construction used MEGA X’s model selection tool, with a General Time Reversible Model (GTR) selected with discrete gamma distribution (5 categories, G parameter = 0.6923) and allowing for evolutionarily invariable sites (51.83% sites). All three codon positions and noncoding sites were used, with site coverage cut-off 90%, resulting in 615 positions used in the final dataset for tree construction. Neighbour Joining (NJ) tree analysis used the p-distance method and the same gamma distribution (for rate variation), site coverage cut off, and codon positions as ML. Phylogeny of ML and NJ trees was tested with the bootstrap method (1,000 replicates).
RESULTS
PHYLOGENETIC ANALYSES
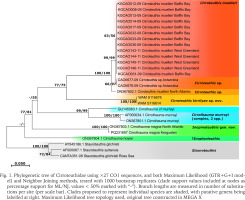
Molecular phylogeny of CO1 data for cirroteuthid and stauroteuthid taxa (27 sequences) (using stauroteuthids as an outgroup; per Taite et al. 2023, Verhoeff 2023b) revealed several well-supported clades and undescribed taxa (Fig. 1). Within Cirroteuthidae Cirroteuthis formed a well-supported clade (77%/75% bootstrap support, ML/NJ respectively) with three or four putative taxa, 13 sequences for Cirroteuthis muelleri from off west Greenland were near identical (clade 63%/56% support) and sister to two putative Cirroteuthis sp. sequences from Antarctica (clade 99% support), which formed a clade (100% support) with a more distantly related Cirroteuthis sp. sequence from the North Atlantic (mid-Atlantic Ridge), with Cirroteuthis kirrilyae sp. nov. being sister to all other Cirroteuthis sequences. These Cirroteuthis sequences formed a larger clade with Cirrothauma murrayi sequences (though only well supported in NJ analysis, 55% support), the latter comprising two species (a C. cf. murrayi (from the North Atlantic) being distinct from two other C. murrayi sequences from both the Atlantic and Pacific (together forming a clade with 99% support)). Basal to the Cirroteuthis + Cirrothauma murrayi-like clade were three other sequences; two closely related sequences for “Cirrothauma” magna (North Atlantic and Indian Ocean) were sister to Cirrothauma murrayi and Cirroteuthis sequences (clade 77%/81% support); basal to all other Cirroteuthidae sequences was a “Cirrothauma” sp. sequence from the NE Pacific (tentatively assigned to “C.” hoylei given specimen morphology as will be discussed), although its phylogenetic position was uncertain (low bootstrap support). Because “C.” magna and “C.” hoylei are united by shared morphology, they are allocated to a new genus in the following taxonomy section (Inopinoteuthis gen. nov.), even though phylogenetic relationship between these taxa remains uncertain using CO1 data.
Fig. 1
Phylogenetic tree of Cirroteuthidae using ×27 CO1 sequences, and both Maximum Likelihood (GTR+G+I model) and Neighbor Joining methods, tested with 1000 bootstrap replicates (clade support values included at nodes as percentage support for ML/NJ, values < 50% marked with “-”). Branch lengths are measured in number of substitutions per site (per scale bar). Clades proposed to represent individual species are shaded, with putative genera being labelled at right. Maximum Likelihood tree topology used, original tree constructed in MEGA X
SYSTEMATICS
Class CephalopodaCuvier, 1795
Order OctopodaLeach, 1818
Suborder CirrataGrimpe, 1916
Superfamily CirroteuthoideaKeferstein, 1866 (fide Verhoeff 2023b)
Diagnosis. Cirrates with secondary webbing (between primary web and arms), elongate cirri (length > 4–10× maximum sucker ⌀), and sepioid gills. Optic nerves in single bundle through white body; digestive gland entire; radula and posterior salivary glands absent (Verhoeff 2023b).
Families. Cirroteuthidae and Stauroteuthidae.
Family Cirroteuthidae Keferstein, 1866
Diagnosis. With shell located dorsally over viscera; saddle greatly thickened; lateral wings short, broad along antero-posterior axis, resembling a saddle or butterfly when viewed dorsally.
Genera. Cirroteuthis, Cirrothauma, and Inopinoteuthis gen. nov.
Remarks. The shell “median saddle” (bridging the lateral wings where fin muscles attach) is greatly thickened; the lateral wings extend ventrally either side of the viscera, and variably expand antero-posteriorly. The saddle-shaped shell has the anterior and posterior expansion of the lateral wings being more equal, and the overall shape of the shell (viewed dorsally) is longer than wide. The butterfly-shaped shell is created by a more unequal and greater anterior expansion of the lateral wings, with the overall shell form being broader than long (viewed dorsally) (see illustrations in later taxonomy sections).
Gill lamellae counts seem to separate Cirroteuthidae from Stauroteuthidae; in stauroteuthids the gills have 8 lamellae per gill (4 per demibranch) (Collins & Henriques 2000, Verhoeff 2023a), while members of Cirroteuthidae have approximately double the lamellae count, Cirrothauma murrayi with 7–9 lamellae per demibranch (14–18 per gill) (Aldred et al. 1983), Cirroteuthis have ~7 lamellae per demibranch (~14 lamellae per gill) as detailed herein, while Inopinoteuthis (I. magna) have 5 or 6 per demibranch (~10–12 per gill) (Guerra et al. 1998).
Genus Cirroteuthis Eschricht, 1836
Sciadephorus Reinhardt et Prosch, 1846
Bostrychoteuthis Agassiz, 1846: p. 50.
Diagnosis. Shell saddle-shaped, arms 2–3× ML, web nodule present on each ventral arm edge. Arm sucker counts ~28–39 on longest arms; mid arm suckers with vestigial acetabulum, atop short fleshy stalks; proximal and distal suckers with acetabula well-developed and without stalks. Suckers and cirri absent from arm tip distal to webbing; arm tip filamentous.
Type species. Cirroteuthis muelleri Eschricht, 1836 by original designation.
Cirroteuthis kirrilyae sp. nov.
Figs 2–12
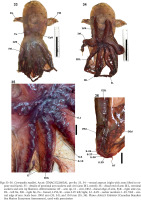
Cirroteuthis kirrilyae sp. nov. body and arm details: 2, 3 – male (WAM S116614), pre-fix (dorsal and ventral aspects, respectively); 4 – female (MV F245713), pre-fix (photography Karen Gowlett-Holmes, used with permission); 5 – preserved specimen (MNHN-IM-2022-18008); 6 – proximal suckers (WAM S116688); 7–10 – mid-arm suckers (WAM S116688, 6, 7; MV F245713, 8, 9); 11, 12 – distal arm suckers and web nodule, female (10, MV F245713; J, WAM S116614). Abbreviations: Acet – sucker acetabulum, Ci – cirrus, Ey(L) – left side eye, Fu – funnel, I–IV R – arms I–IV (right side), Inf – sucker infundibulum, Nd – nodule, St – stalk. Scale bars: 50 mm (2–5); 5 mm (6, 7, 11); 2 mm (9, 10)

Figs 13–20
Organs, Cirroteuthis kirrilyae sp. nov.: 13 – mantle cavity (WAM 116688); 14, 15 – digestive system (female NMNZ M.109379), rotated aspects left–right (14), and redrawn from O’Shea (1999) (15); 16, 17 – beaks, upper and lower (16, 17 respectively) (NMNZ M.109379), redrawn from O’Shea (1999); 18, 19 – male reproductive system (WAM 116688), rotated aspects; 20 – female reproductive system (NMNZ M.109379) rotated aspects. Abbreviations: AGC – accessory gland complex, ASG – anterior salivary glands, BB – buccal bulb, Bk – beak, Ca – caecum, DG – digestive gland, DO – distal oviduct, DOG – distal oviducal gland, Du – duct (hepatic duct), Fu – funnel, GiL/R – gill left and right side, Hd – hood, Int – intestine, LWa – lateral wall, LWi – lateral wing, Oes – oesophagus, OO – olfactory organ, Ov – ovary, PO – proximal oviduct, POG – proximal oviducal gland, Re – rectum, Ro – rostrum, SC1–3 – spermatophoric complex parts 1–3, SS – spermatophoric sac (Needham’s sac), St – stomach, Te – testis, TO – terminal organ, VD – vas deferens. Scale bars: 10 mm (13); 5 mm (14–20). Photo: 14, 20 – Kerry Walton, NMNZ, used with permission
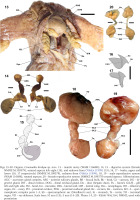
Figs 21–31
Internal shell, Cirroteuthis kirrilyae sp. nov.: 21–23 – male (WAM S116614), dorsal (21), lateral (22), and anterior end-on (23) aspects; 24, 25 – female shell dorsal (24) and ventral aspects (25) (NMNZ M.109379); 26–28 – shell from specimen MNHN-IM-2022-18008, lateral (26), dorsal (27), and ventral (28) aspects; 29–31 – shell from USNM 817334, dorsal (29), ventral (30), and lateral (31) aspects. Abbreviations: AnEd – anterior edge (of lateral wing), DEd – dorsal edge (of shell), LW – lateral wing, PoEd – posterior edge (of lateral wing), Sa – saddle, VEd – ventral edge (of shell). Scale bars: 5.0 mm. Photo: 24, 25 – Kerry Walton, NMNZ; 29-31 – Michael Vecchione, NMNH, used with permission
Fig. 32
Cirroteuthis kirrilyae sp. nov. capture localities. Red circles, specimens examined by authors; “?” badly damaged RV “Eltanin” specimen USNM 817334 attributed to Cirroteuthis ?kirrilyae sp. nov.; black square, identification by Nesis (1987) (not examined)
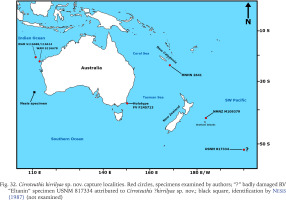
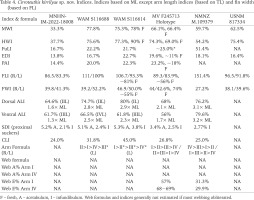
Table 4
Cirroteuthis kirrilyae sp. nov. Indices. Indices based on ML except arm length indices (based on TL) and fin width (based on FL)
urn:lsid:zoobank.org:act:6DF6704F-2171-4DA7-A536-2008945B62CE
Cirroteuthis n. sp. A – Nesis 1987: p. 283.
Cirroteuthis muelleri – Voss & Pearcy 1990: pp. 51–57.
Cirroteuthis cf. muelleri – O’Shea 1999: pp. 62–66; Verhoeff 2022: pp. 205–211; Ziegler & Sagorny 2023: pp. 3–7.
Material examined
Holotype: MV F245713 (female; off East Gippsland, Tasman Sea, 37°47.52′–49.07′S, 150°22.92′–21.18′E, 2,338−2,581 m; RV “Investigator”, IN2017_V03, Stn 035, 25/V/2017).
Paratypes: WAM 116688 (female; off Low Point, Indian Ocean, 20°48.14′S, 111°36.93′E, 2,013 m; RV “Investigator”, IN2022_V09, Stn 024, 3/XII/2022); WAM 116614 (male; off Low Point, Indian Ocean, 20°48.14′S, 111°36.93′E, 2,013 m; RV “Investigator”, IN2022_V09, Stn 024, 3/XII/2022 [with molecular data]); MNHN-IM-2022-18008, former MNHN 2641 (sex undetermined; Norfolk Ridges, western Pacific, 23°51.50′–56.42′S, 168°25.98′−28.34′E, 1,846−1,862 m; RV “Tangaroa”, HALIPRO 2, Stn BT82, 22/XI/1996); NMNZ M.109379 (female; Chatham Rise, southwestern Pacific, 42°36.79′S, 176°09.81'W, 1,999−2,002 m; RV “Tangaroa”, TAN9202, Stn 149, 2/III/1992).
Comparative material:
Cirroteuthis kirrilyae sp. nov., WAM 116678 (sex undetermined; off Point Edgar, Indian Ocean, 22°22.47′S, 113°13.48′E, 1,497 m; RV “Investigator”, IN2022_V09, Stn 006, 25/XI/ 2022 [badly damaged juvenile, with molecular data]); Cirroteuthis ?kirrilyae sp. nov., USNM 817334 (sex undetermined; southwestern Pacific, 51°50.00′S, 159°50.00'W, 4,621–4,667 m; RV “Eltanin”, Expedition USAP, EL14, Stn 1184, 3/VIII/1964 [Photography provided by NMNH]); Cirroteuthis muelleri, TMAG E22665A (Amundsen Gulf, Arctic Ocean off Northern Canada, 70°33.60'N, 122°54.90'W, 640 m; AMG_03, 2021); TMAG E22665B (Canadian Beaufort Sea, Arctic Ocean off Northern Canada, 71°15.02'N, 135°07.81'W, 1,000 m; KUG_07, 2017).
Diagnosis. A Cirroteuthis with 36–39 suckers on at least some arms; with web nodule on dorsolateral arm margin at level of sucker 30–35, gills with 7 or 8 lamellae per demibranch.
Description
Fresh: Mantle antero-posteriorly elongate; posteriorly shorter on male (Figs 2, 3) than females (Fig. 4); ML ~36–40% of TL. Mantle and head width similar; MWI 66–78%, HWI 69–90%. Fins positioned midway between head and mantle apex; of moderate length (FLI 56–81%), broad (FWI 55–74%), with narrow base; posterior edge near-straight, widest ~midway along length, with distinctly rounded anterior and distal margins. Eyes slightly ventro-lateral, EDI ~11%. Pallial aperture narrow, closely enveloping funnel (PAI ~18%).
Preserved: Mantle shrunken especially posterior to fin bases (giving more squared posterior face) (Fig. 5). ML 24–39% TL; mantle and head width similar, MWI 33–78%, HWI 38–77%. Fin bases terminal (because of posterior mantle shrinkage); fins long, broad, FLI 83–151%; FWI 27–52%, narrowing basally, widest ~midway along length, anterior and distal margins rounded, posterior margins near straight (Figs 2–4). Eyes slightly ventrolateral, EDI 14–23%; pallial aperture narrow, closely enveloping funnel (PAI 14–23%). Funnel long, FuLI 17–51%; funnel organ apparent on one specimen (MV F245713) but distorted, ~W-shaped and with lateral limbs broad, rounded, and fleshy, funnel organ on another specimen (WAM S116688) with only a faint U-shaped scar marking its location. Olfactory organs small, ovoid.
Arms I and II 65–80% TL (1.6–3.1× ML), and III and IV 56–80% TL (1.3–3.2× ML); arm formula variable, ~I>II>III>IV (Figs 2–5). Arms with elongate fleshy web nodule on ventrolateral margin, near arm tip between suckers 30 and 35 (arms I), suckers 29 and 33 (arms II), suckers 28 and 31 (arms III), and suckers 28 and 29 (arms IV) (Figs 11, 12). Primary web extensive, damaged on all arms; secondary web obliterated on most specimens, present along outer arm face (most intact on female MV F245713), extending from near arm base to or proximal to nodule (separating primary web from arm except for points proximal and distal to attachment). Primary web attached to web nodule on ventrolateral arm edge, and to dorsolateral arm edge near the terminal sucker (5–7 suckers after nodule). Web sectors B–D asymmetrical, sectors A and E symmetrical; on MV F245713 the outer primary web edge in sector E was intact between web nodules, with medial depth ~70% arm IV.
ASC to 36–39 suckers (or remnants of) on arms I (other arms with ~28–39 suckers), differentiated into proximal- (suckers 1–8 or 9), mid- (suckers 9 or 10–31), and distal- (suckers 25–32 to 36–39) types. Beyond the terminal sucker bud, arm tips taper to filaments (6–10 mm long); filaments devoid of suckers, cirri, and webbing. Proximal-arm suckers gradually increase in size, with numbers 3, 4, or 5 largest (Fig. 6), before slightly decreasing in size. Proximal suckers with rounded acetabulum, with bases almost adjoining or with ~1–2 infundibulum spaces between them, elevated from arm surface, exceeding infundibular ⌀ (SDI 3.4–5.3% acetabulum, 2.1–3.8% infundibulum); infundibulum with thick ring, surrounding small pad and aperture. Mid-arm sucker ⌀ decreases from suckers 8–10, with the spacing between them (3–5 infundibulum spaces) increasing (Figs 7–10). Mid-arm suckers with thick infundibular ring, but with pad and aperture reduced to shallow pit (Fig. 9), infundibulum SDI 1.3–2.3%; acetabulum highly reduced to a shallow cup below infundibular structures (often embedded in stalk but exposed in Fig. 10); mid-arm suckers on variably developed fleshy stalks or pedestals (Figs 7–10), some almost flush with arm surface, others elevated 2–3× sucker ⌀ from it). Distal-arm suckers commence further along dorsal arms (from sucker 25–32 to 36–39, the terminal suckers becoming small and bud-like); suckers urn-like with rounded, well-developed acetabulum chamber (exceeding infundibulum ⌀) (Figs 11, 12); infundibular structures similar to proximal-type suckers, with small but distinct aperture. Female (MV F245713) with distal-type suckers intermediate in size to proximal- and mid-arm sucker types (largest 1.4 mm acetabular ⌀; SDI 2.5% acetabular ⌀, 1.7% infundibular ⌀), with proximal 5 or 6 largest, and with acetabulum swollen (Fig. 11), reducing distally to small buds; male (WAM S116614) with distal-type sucker ⌀ similar or slightly smaller than mid-arm suckers (0.65 mm acetabular ⌀; SDI 2.2% acetabular ⌀, 1.7% infundibular ⌀), with acetabulum bulbous (Fig. 12). Distal suckers well-spaced, bases not touching, separated by ~0.5–1 acetabulum spaces.
Cirri commence as minute buds between suckers 2 and 3; cirrus length initially increases gradually, then abruptly between suckers 7 and 12 (WAM S116688 cirrus length on arm IIL 6.0 mm (between suckers 9 and 10), 13.7 (between suckers 10 and 11), 14.3 (between suckers 11 and 12); WAM S116614 cirrus length on arm IL 8.4 mm (between suckers 9 and 10), 9.8 (between suckers 10 and 11), and 13.5 mm (between suckers 11 and 12); MV F245713 cirrus length 4–6 mm to ~15 mm (between suckers 7 and 10)). Longest cirrus occurs at ~30% arm length (or slightly more proximal), greatest cirrus length CLI 24–45% (4.6–9.4× acetabulum ⌀, 9.0–12.8× infundibulum ⌀), between sucker ~10–12 (Fig. 7); cirrus lengths very gradually decrease more distally, before rapidly decreasing in length in the distal-type sucker field (Fig. 12); terminal-most cirri minute, bud-like, terminating 2–5 suckers prior to the distalmost sucker bud1.
Gills “sepioid”, each with 7 or 8 lamellae per demibranch (~14–16 primary lamellae per gill); with proximal and terminal lamellae smaller; terminally with 2 or 3 small lamellae sharing common base (Fig. 13).
Optic lobes spherical with single optic nerve bundle penetrating white body; white bodies well separated, approximately spherical, of similar size to eye.
Digestive system (NMNZ M.109379) unremarkable (Figs 14, 15); buccal bulb large; anterior salivary glands prominent; radula and palatine teeth absent; oesophagus dilated distally into a crop (without diverticulum); stomach and non-coiled caecum of comparable size; digestive gland without lobes; intestine short, of comparable length to oesophagus, slightly dilated for distal half; rectum slightly projecting into mantle cavity near funnel base, its epithelium dark-purple. Beaks as described by O’Shea (1999: p. 63) (Figs 16, 17); upper beak tall (height 86% length), hood moderately deep (depth 68% beak length), jaw cutting edge without teeth, rostrum pointed, deflexed orally, lateral walls with rounded crest; lower beak moderately tall (height 64% width), lateral walls with broadly concave basal notch and rounded crest, hood shallow (hood length 50% beak length), rostrum acutely pointed, lateral wings long (length 100% beak length) with weak folds.
Male reproductive system (Figs 18, 19) with small testis; vas deferens shorter than tripartite spermatophoric complex (SC2 and 3 larger); spermatophoric sac (Needham’s sac) at base of accessory gland complex (AGC); AGC large, disc-like, with faint subdivisions; terminal organ very short.
Oviducal gland of mature female (WAM S116688) (Fig. 13) with two hemispheres – the distal brownish, striate, and ~80% larger than pale-beige-coloured proximal hemisphere. Ovary sac of immature female (NMNZ M.109379) (Fig. 20) with immature oocytes; proximal oviduct narrow, elongate (of comparable length to combined lengths of oviducal gland and distal oviduct); oviducal gland broadly spherical and striate, with proximal hemisphere marginally longer (56%) than distal hemisphere, both hemispheres beige-coloured; distal oviduct short (~55% oviducal gland length).
Shell (Figs 21–31) dorsal; shell wings broadly expanded, with outer faces converging somewhat posteriorly (posterior shell width ~40–80% anterior width) (Figs 21, 27, 29), wing lateral profile ovoid (Figs 22, 26, 31), with wings slightly broader at anterior limits; overall shell longer than wide, width ~80–100% shell length; shell saddle (median point where lateral wings meet) thick, almost spanning the full depth of shell dorsoventrally (Fig. 23), positioned ~40–50% along shell anteroposterior axis. All shells variably distorted.
Pigmentation in fresh condition (Figs 2–4) orange to red over mantle, head, and arms (lighting may have influenced the apparent colour); where skin has abraded on fins and parts of mantle, paler (translucent) subepithelial tissue is apparent; internal structures (e.g., fin muscles, gills) are visible in translucence. Oral surfaces of arms and remnant web with darker red epithelium (more purple–maroon on preserved material); suckers and cirri light beige, contrasting with dark pigmented aboral arm and sucker stalk epithelia (Figs 5–12).
Type locality. Off East Gippsland, southeastern Australia, Tasman Sea (37°47.52′–49.07′S, 150°22.92′–21.18′E), 2,338−2,581 m depth.
Recognised distribution. Known from off southeastern and northwestern Australia, New Zealand, and New Caledonia, 1,497–2,581 m (Fig. 32). Potential records are from southwestern Australia (Naturaliste Plateau, black square, Fig. 32) (Nesis1987: p. 283), and southeast of New Zealand (denoted “?” on Fig. 32).
Etymology. The species is named after Dr Kirrily Moore (collection manager, invertebrate zoology, TMAG) in recognition of her support and encouragement to the lead author, without which this work would not have been possible.
Remarks. The immaturity of available Cirroteuthis kirrilyae sp. nov. specimens suggests that this species attains a larger size. Because arm sucker count usually increases with arm length (Toll 1988), higher ASC values are not unexpected on more mature specimens. In this case, this species will differ even more demonstrably from the type species, which is also the only other recognized species in the genus.
Gilbert Voss attributed one damaged specimen (arms and webbing mostly destroyed, and shell damaged) (USNM 817334) from the southwestern Pacific to “Cirroteuthis sp.”. The shell is typical of Cirroteuthis (Figs 29–31), but because the specimen is extensively damaged, and caught considerably deeper and further south than any other specimen herein attributed to Cirroteuthis kirrilyae sp. nov., we only tentatively attribute it to species. The repository of a further specimen referred to by Nesis (1987: p. 283) as “Cirroteuthis n. sp. A” from the Naturaliste Plateau, southwestern Australia (uppermost abyssal depth), is unknown. This unillustrated specimen was ~20–30 cm TL, had arms just over 50% TL, and mid-arm suckers that differed from proximal and distal suckers (“suckers in median parts of arms high, barrel-like, with tiny openings, in most proximal and distal parts suckers conical”2). While plausibly conspecific with Cirroteuthis kirrilyae sp. nov., we do not attribute it to this species because there are too few cirrates in collections from these waters and depths to make an informed judgement.
Molecular data (Fig. 1) indicates that Cirroteuthis kirrilyae sp. nov. is distinct from Cirroteuthis muelleri (s.s.) from the Arctic (and far-northern Atlantic). Additionally, sequences from multiple C. muelleri off western Greenland are distinct from Cirroteuthis sp. specimens from the Atlantic and off Antarctica. A large (ML 173 mm) Arctic Ocean (Amundsen Gulf) Cirroteuthis muelleri (TMAG E22665) has 32 suckers on its dorsal arms and 29 or 30 on its ventral arms (Figs 33–36). Eschricht (1836) described the type of Cirroteuthis muelleri with 30 suckers per arm. Robson (1932) also reported ASC values of 30 per arm for this species. Knudsen & Roeleveld (2002) (translation of Reinhardt & Prosch 1846) provided more detailed sucker counts for two large (TL 288, 229 mm) Arctic specimens collected proximal to the type location; for one (TL 288 mm) there were 31 or 30 suckers (arms I), 30 (II), 29 (III and IV), and for the other (TL 229 mm) there were 34 or 33 suckers (arms I), 33 or 32 (II), 29 or 30 (III), 29 (IV). Pacific Cirroteuthis kirrilyae sp. nov. consistently has higher sucker counts (to 36–39 per arm). Web nodules also occur more distally on Cirroteuthis kirrilyae sp. nov. (between suckers 30–35 on dorsal arms), compared with Cirroteuthis muelleri (~sucker 25 per Eschricht (1836), about 25–27 per Robson (1932)).
Figs 33–36
Cirroteuthis muelleri, Arctic (TMAG E22665A), pre-fix: 33, 34 – ventral aspects (right with arms lifted to expose oral faces); 35 – details of proximal arm suckers and cirri (arm III L noted); 36 – distal end of arm III L, terminal suckers and arm tip filament. Abbreviations: AT – arm tip, Ci – cirri, DEd – dorsal edge of arm, EyR – right side eye, FiL – left fin, FiR – right fin, Fu – funnel, I–IVL/R – arms I–IV left/right, S.1–S.29 – sucker numbers 1–29, VEd – ventral edge of arm. Scale bars: 100.0 mm (33, 34), and 10.0 mm (35, 36). Photo: Ashley Ehrman (Canadian Beaufort Sea Marine Ecosystem Assessment), used with permission
As illustrated (Figs 21–31), the shell of Cirroteuthis kirrilyae sp. nov. is variable, seemingly from preservation shrinkage and artefacts. While O’Shea (1999) referred to the shell of the New Zealand specimen (NMNZ M.109379) as being “vacuolated,” as he did with others attributed to the genera Opisthoteuthis, Luteuthis, and Cirroctopus, this is now regarded to be an artefact of freezing (which was the main way that material was delivered to him prior to his formalin-fixing it), as no such vacuolation has been observed in specimens fixed fresh (without having been frozen). In any case, while the shell in this genus is diagnostic, the variability in its form that we attribute to the effects of preservation renders it less ideal for species-level comparison.
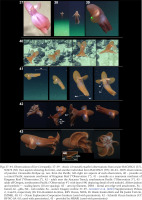
Although aspects of the shell, internal anatomy, arm sucker counts, and web-nodule positions (based on illustrations) of specimens attributed by Voss & Pearcy (1990) to Cirroteuthis muelleri from the abyssal basin of the northern Pacific are consistent with Cirroteuthis kirrilyae sp. nov., it is hard to confirm them as conspecific without examining material. Remotely operated vehicle (ROV) observations of animals from off Oregon (northeastern Pacific) are visually similar to those from the southern and central Pacific. ROV’s have documented Cirroteuthis across the Arctic and Pacific, allowing comparison of Arctic Cirroteuthis muelleri (Figs 37–39) with putative Cirroteuthis kirrilyae sp. nov. (Figs 40–44) from the central, northeastern, and southeastern Pacific. These observations emphasise morphological features lost on preserved specimens – most notably the very elongate posterior mantle (filled with highly gelatinous tissue), and the strongly rounded distal ends of the fins. These ROV observations further reveal differences in pigmentation between the ‘sensu stricto’ Arctic Cirroteuthis muelleri, and Pacific animals herein attributed to Cirroteuthis kirrilyae sp. nov., assuming that only two species are involved. Arctic specimens are more pink, paler over the mantle and fins, and especially around the head, and slightly darker pink-red on the arms and web (Figs 37–39). Putative Cirroteuthis kirrilyae sp. nov. is more uniformly pigmentated, orange–brown tones being predominant; although we cannot correct for lighting, two juveniles were orange (Figs 40–41) and two adults were darker–brown (Figs 42–44) over the mantle, fins, head, arms and webbing (the only areas of reduced pigment were around the eyes and the proximal area of arms and webbing orally) (Fig. 43, right-most). Imagery of larger northeastern and southeastern Pacific animals reveals ~7–9 suckers distal to the nodule towards the arm tips, consistent with the great number of distal suckers that we describe for Cirroteuthis kirrilyae sp. nov.
Figs 37–44
Observations of live Cirroteuthis: 37–39 – Arctic Cirroteuthis muelleri observations from cruise HACON21 (37), SO276 (38) (two aspects showing fin form), and another individual from HACON21 (39); 40–44 – ROV observations of putative Cirroteuthis kirrilyae sp. nov. from the Pacific, left–right are aspects of each observation, 40 – juvenile at a central Pacific seamount northwest of Kingman Reef (“Observation 1”), 41 – juvenile at a seamount northeast of Kingman Reef (“Observation 2”), 42 – adult near the Atacama Trench, southeastern Pacific (“Observation 3”), 43 – adult off Oregon, northeastern Pacific (“Observation 4”) with inset (44) depicting detail of web nodule). Abbreviations and symbols: * – scaling lasers (10 cm spacing), AT – arm tip filament, DWA – dorsal arm edge wed attachment, Fu – funnel, Gi – gills, Nd – web nodule, Su – sucker. Imagery credits: 37–39 – Golikov et al. (2023) Supplementary Videos 2, 4 and 8, respectively (Dr Eva Ramirez-Llodra, REV Ocean, NIVA; Dr Habil Saskia Brix and Dr James Taylor, DZMB), 40, 41 – Ocean Exploration Cooperative Institute (used with permission), 42 – Schmidt Ocean Institute (CC BY-NC-SA 4.0; used with permission), 43 – provided by MBARI (used with permission)
Genus Cirrothauma Chun, 1911
Diagnosis. Cirroteuthids with “butterfly shaped” shell, long arms (3–4× ML), no web nodules, suckers extending to arm tips, vestigial eyes (embedded in head, without opening, lacking lenses). Suckers vestigial (shallow infundibular dish without acetabulum), proximal 5–8 sessile, atop small mounds, but with remainder atop elongate fleshy peduncles (stalks) of length ~5–6× sucker ⌀ (modified from Aldred et al. 1983).
Type species. Cirrothauma murrayi Chun, 1911 by original designation.
Remarks. As recognised herein, Cirrothauma contains the type species and one undescribed species. Because molecular evidence indicates that Cirrothauma murrayi and “Cirroteuthis” magna (only recently attributed to Cirrothauma) do not form a well-supported clade (no more so than “Cirroteuthis” magna does with Cirroteuthis muelleri), we are compelled to describe a new genus to accommodate “Cirroteuthis” magna. On grounds of similarity, we provisionally attribute “Cirroteuthis” hoylei to this new genus also.
Genus Inopinoteuthis gen. nov.
urn:lsid:zoobank.org:act:B1C5C992-3DB5-4F01-9D39-7958D687A290
Diagnosis. Cirroteuthids with “butterfly shaped” shell, with long arms (3.5–5.0 ×ML), no web nodule, well-developed eyes, high ASC (~50–60), with suckers extending to arm tips; suckers having well-formed acetabulum embedded into arm or born from it on short stalks in the mid-arm region (modified from Guerra et al. 1998, Collins & Villanueva 2006).
Type species. Cirroteuthis magna Hoyle, 1885
Etymology. From the Latin “inopinus” for unexpected, in reference to the unexpected genetic placement of the type species relative to Cirrothauma (sensu stricto) (C. murrayi).
Remarks. Inopinoteuthis differs from Cirrothauma in possessing well-developed eyes (with lenses and eye openings) and in sucker morphology. Unlike Cirrothauma murrayi, the suckers of I. magna and I. hoylei have distinct acetabular chambers and short/thin or no stalks; for I. magna Guerra et al. (1998: fig. 4b, fig. 9b) illustrated some proximal arm suckers on one specimen placed on short and thin stalks (no longer than sucker diameter), and some distal suckers on another specimen with string-like stalks attaching laterally on the sucker (though this could be attributed to damage, in that the suckers may have been partially detached). Mid- and distal-arm suckers of I. magna were illustrated with a distinct acetabulum, similar to that of I. hoylei herein (see Figs 50–52 for distinction between Cirrothauma and Inopinoteuthis). The shell of Inopinoteuthis is similar to that of Cirrothauma (Figs 46–49), but differs from it in having more rounded wing ends.
Figs 45–52
Shell and sucker morphology of Cirroteuthis, Cirrothauma, and Inopinoteuthis gen. nov. (shells viewed dorsally, with posterior edge at top): 45 – shell of Arctic Cirroteuthis muelleri (re-drawn from Bizikov 2004), 46 – shell of Cirrothauma murrayi (re-drawn from Aldred et al. 1983: fig. 9b); 47 – shell of Inopinoteuthis magna holotype (Hoyle 1886); 48 – I. magna (re-drawn from Guerra et al. 1998: fig. 4c); 49 – I. hoylei holotype shell (Hoyle 1886); 50 – Cirrothauma murrayi schematic of mid-arm vestigial sucker and stalk (re-drawn from Aldred et al. 1983: fig. 28); 51 – photograph of C. murrayi vestigial suckers, stalks, and cirri (photo courtesy of Michael Vecchione, Tree of Life Web project, ID 10541; CC BY-NC 3.0); 52 – Inopinoteuthis magna suckers (re-drawn from Guerra et al. 1998: fig. 4b & fig. 9b). Abbreviations and symbols: * – putative stalk or damaged string of tissue, Ac – acetabulum, Ci – cirrus, Inf – infundibulum, LW – shell lateral wig, nv – nerve, Sa – shell saddle, St – stalk
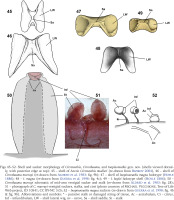
Digestive anatomy may also serve to differentiate Inopinoteuthis gen. nov. from Cirrothauma. The bag-like caecum of I. magna is twice the size of the stomach (Guerra et al. 1998: figs 5a, 10a, O’Shea 1999: fig. 46E), whereas in Cirrothauma murrayi it is only half as large as the stomach (Aldred et al. 1983: fig. 25).
Inopinoteuthis magna (Hoyle, 1885) comb. nov.
Cirroteuthis magna (in part) – Hoyle 1885: pp. 109, 233; Hoyle 1886: pp. 56–61, plate XII, figs 1–7, plate XIII, figs 1, 2; Robson 1932: pp. 162–164; Guerra et al. 1998: pp. 51–81; Collins et al. 2001a: pp. 357–358, fig. 1.
Cirrothauma magna (Hoyle) – O’Shea 1999: pp. 66–68, figs 45, 46.; Verhoeff 2023a: p. 163, fig. 7.
Brief description (derived from: Guerra et al. 1998, Collins et al. 2001a)
Large (TL to 1.7 m), mantle rounded posteriorly, MWI 37–77% , head slightly narrower than mantle, HWI 48–64%; fins lateral, paddle-shaped, with posterior edge near straight, distal end pointed, basally constricted, large (FLI 71–114%, FWI 38–50%). Eyes large (EDI 24–43%); funnel closely enveloped by narrow aperture (FuLI 23–43%; PAI 10–14%); gills sepioid, with 5 or 6 lamellae per outer demibranch. Arms elongate (73–79% TL), slender, no consistent arm formula (but often with dorsal arms slightly longer). ASC to ~75 (from illustration), suckers small (greatest SuDI 4.1–4.6%); proximal suckers (to sucker 29) closely spaced with ~cylindrical acetabulum; following mid-arm suckers with short/narrow stalks, rounded acetabulum; distal arm suckers (over distal arm third) sessile, with more muscular and ‘barrel-like’ acetabulum. Cirri commence between suckers 4 and 5, longest cirri ~half way along arms (CLI 33–61%, 8–14× greatest sucker ⌀), decreasing in length rapidly in distal arm half; cirri apparently terminating near points of web attachment.
Internal shell “butterfly-shaped” (Figs 47–48, 58–59). Digestive system with large buccal bulb (beaks and rest of digestive tract illustrated by Guerra et al. 1998: figs 5, 10), and odontophore (without radula); anterior salivary glands paired, large; oesophagus with simple crop, stomach ovoid; caecum large, bag-like (double stomach dimensions); intestine ~1.5× oesophagus length; digestive gland without lobes. Male reproductive system (Guerra et al. 1998: fig. 6) with AGC as a singular large disc-like mass with short terminal organ; SC (or “seminal vesicles”) tripartite, elongate. Female with short proximal oviduct (equal or less than distal oviduct length); oviducal gland unremarkable, bipartite, longitudinally striate.
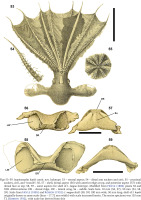
Figs 53–59
Inopinoteuthis hoylei comb. nov. holotype: 53 – ventral aspect; 54 – distal arm suckers and cirri; 55 – proximal suckers, cirri, and “mouth”; 56, 57 – shell, dorsal aspect (56) with anterior edge at top, and posterior aspect (57) with dorsal face at top; 58, 59 – same aspects for shell of I. magna holotype. Modified from Hoyle (1886) plates XI and XIII. Abbreviations: DR – dorsal ridge, LW – lateral wing, Sa – saddle. Scale bars: 10 mm (56, 57), 50 mm (53, 58, 59). Scale from Hoyle (1885) and Robson (1932): I. magna shell (58, 59) 100 mm wide, 50 mm long; shell of I. hoylei originally drawn to same scale (thus ~17.72 mm width) with scale increased herein. The entire specimen was 155 mm TL (Robson 1932), with scale bar derived from this
Inopinoteuthis hoylei (Robson, 1932) comb. nov.
Figs 60–66
Inopinoteuthis hoylei comb. nov. holotype: 60 – oral aspect of arm crown and buccal structures; 61, 62 – proximal suckers and cirri, arm IIL (61) and arms IIIL and IVL (62); 63, 64 – mid-arm suckers and cirri; 65 – distal arm suckers and cirri, arm IVL; 66 – buccal bulb and adjacent parts of brain, with salivary glands and ganglia. Abbreviations/symbols: * – scar marking detached sucker, Ac – acetabulum, ASG – anterior salivary glands, BB – buccal bulb, BG – buccal ganglia, Br – brain, IL/R, IVL/R – arm I left/right, arm IV left/right, Inf – infundibular ring, LCi – longest cirri, Oes – oesophagus, OL – optic lobe, Pr.Ci – proximal cirri, S.5, S.10, S.25 – sucker number 5, 10, 25 etc., St – stomach, Su-M – sucker mid-arm. Scale bars in mm marks (with photography). Photo: Kevin Webb, copyright Natural History Museum, London (used with permission)
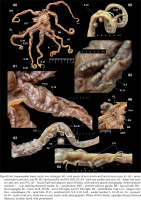
Figs 67–72
Provisional identification of a specimen of Inopinoteuthis hoylei comb. nov. from the northeastern Pacific (FMNH 309245): 67 – ventral aspect; 68 – mantle cavity (digestive gland has been displaced to anterior end of cavity); individual outer gill lamellae marked on GiL; 69 – proximal suckers and cirri; 70 – mid-arm suckers and cirri and transition to distal-arm suckers (black lines mark sucker positions between S.26 and S.34); 71 – distal-arm suckers; 72 – arm tip. Abbreviations: Ci – cirrus, DG – digestive gland, EyR – right side eye, Fu – funnel, GiR/L – gill (right / left side), S.15, 40, etc. – sucker number 15, 40 etc. Scale bars: 50 mm (67), 10 mm (68), 5 mm (69). Photo: Janet Voight, Field Museum of Natural History (used with permission)

Cirroteuthis magna (in part) – Hoyle 1886: pp. 56–61, plate XI, figs 3–4, plate XIII, figs 3, 4.
Cirroteuthis (?) hoylei – Robson 1932: pp. 161–162.
Type material
Holotype: BMNH 1890.1.24.2 (sex indet., ML 32.6 mm; off Valparaiso, Chile, southeastern Pacific, 34°07.00′S, 73°56.00'W, 418 m; RV “Challenger”, Stn 298, 17/XI/1875 [Photography provided by BMNH]).
Comparative material:
?Inopinoteuthis hoylei, FMNH 309245 (sex undetermined, ML ~67 mm: Gorda Ridge, northeastern Pacific, 42°45.356'N, 126°42.574'W, 2,741 m; RV “Atlantis”, DSV-2 “Alvin” submersible, dive 4044 (suction sampler), 31/VIII/2004 [Photography provided]).
Description. Holotype (and only known specimen) incompleteness precludes a detailed description. Characters and their states described for it, or that can be discerned from what remains or has been illustrated, are, however, consistent with the new genus. Because ML cannot be determined, standard indices are approximated from details provided by Robson (1932); absolute measurements and relative dimensions of structures are provided in the text.
Mantle and head largely obliterated; TL 155 mm (fide Robson 1932), with arm length 79% TL (ML ~21% TL, or ~32.6 mm); HW and MW comparable; arms ~3.8× ML. Fins lateral, paddle-shaped (Hoyle 1886; Fig. 53), large (FL 42 mm, FW 10 mm, Robson 1932); FL exceeding ML, FW ~24% FL. Neither the funnel nor gills were remarked upon by Robson (1932).
Arms slender (Figs 53, 60) (measured herein at 97 mm (IL), 95 mm (IIL), 91 mm (IIIR)); the longest at 97 mm being 3× ML of 32.6 mm, somewhat less than the 3.8× indicated by Robson (1932). Webbing obliterated; Hoyle (1886) reconstructed this for illustrative purposes (Fig. 53); web nodules absent (Figs 60, 65). Hoyle’s (1886) illustration of an arm tip (Fig. 54) depicts no web nodule, and for the suckers to extend to the arm tips.
No arm with complete complement of suckers; ASC to 53 (in specimens’ current condition), and possibly ~55–60 from illustrations provided by Hoyle (1886) (Figs 53–55); suckers separated into proximal, mid-arm, and distal types. Proximal-arm suckers, numbers 1–11 or 12, steadily increase in size (largest suckers ~4–8, ~0.87–0.94 mm ⌀, 2.7–2.9% ML), thereafter slowly decreasing in size (~0.85 mm ⌀ by sucker 10) (Figs 61–62; also Hoyle’s 1886 illustration, Fig. 55). Proximal suckers with barrel-like acetabulum, embedded in arm tissue; infundibular ring thick (not clearly differentiated into a pad), of comparable ⌀ to acetabulum, extending well above acetabulum, with large aperture. Mid-arm suckers commence from numbers 12–14, reduced in size (~70–80% ⌀ of proximal suckers), infundibular ⌀ ~0.65–0.67 mm (~1.9–2.1% ML), acetabular ⌀ ~0.69 mm, rounded cup-shaped, imbedded in a small mound of delicate tissue; infundibular ring thick, extending above acetabulum and free from arm tissue, aperture small (Fig. 63). Mid-arm suckers frequently detached, leaving small indentation on arm tissue (Fig. 64). Distal-arm suckers commence at ~sucker number 28 or 29, are largest at ~sucker 30, ⌀ ~0.89–0.91 mm, then progressively decrease in size to arm tip (Fig. 65; also Hoyle’s 1886 illustration, Fig. 54); suckers with barrel-like acetabulum, standing above arm surface, infundibular and acetabular ⌀ comparable, infundibulum ring thick, extending above acetabulum, with large aperture.
Proximal 12–14 suckers more closely disposed, with spaces between them ~0.5–1.0× proximal sucker ⌀ (Figs 61–62). Spacing abruptly increases to ~3.5–4.5× sucker ⌀ (Figs 63–64). Distal ~25% arm length with suckers more closely disposed, < 0.5–1.0 × distal sucker ⌀, often with adjacent suckers touching (Fig. 65).
Cirri commence as short buds between suckers 2 and 5, then slowly increase in length (~2× sucker ⌀ by sucker 9 or 10; Figs 61–62) (consistent with Hoyle’s 1886 illustration, Fig. 55). Longest cirri ~4.3–5.6 mm (~6.2–8.6× mid-arm sucker ⌀, or ~13–17% ML); Robson (1932) described the longest cirri as 14× sucker ⌀ (~9.2 mm). Cirri reduce in length from distal arm sucker ~28), where they are ~1× distal-arm sucker ⌀, and progressively thereafter reduce to minute size (barely discernible or absent for last ~20 suckers).
Shell length (anteroposteriorly) 51% shell width (Fig. 56); saddle thick (or tall) viewed posteriorly (Fig. 57), with dorsal ridge (shell height 52% shell width); lateral wings forming dorsolateral depressions, expanded anteriorly, truncated posteriorly (anterior expansion of lateral wings, anterior to medial ridge, accounting for 68% shell length in anteroposterior axis).
Buccal bulb large; with large ganglia on posterolateral face (Fig. 66); beaks dissected, presumed lost; anterior salivary glands paired, prominent). Remainder of digestive system and reproductive system damaged.
Remarks. The holotype of Inopinoteuthis hoylei comb. nov., collected off Valparaiso, Chile (southeastern Pacific) was one of two specimens attributed by Hoyle (1885) to “Cirroteuthis” magna Hoyle, 1885 (the lectotype of C. magna was collected from the southern Indian Ocean between Prince Edward and Crozet islands) (Hoyle 1886). Robson (1932) designated the Valparaiso syntype of “Cirroteuthis” magna as the holotype for “Cirroteuthis” hoylei. Fortunately Hoyle (1886) figured both specimens, and for the Valparaiso syntype he included a ventral aspect of the whole animal, and close-ups of proximal and distal suckers and cirri (Hoyle 1886: plate XI figs 3–5; labelled as Stauroteuthis; Figs 53–55), and shell form (Hoyle 1886: plate XIII fig. 3, 4; see Figs 56–57). For the “Cirroteuthis” magna lectotype he figured whole aspects and arm details (Hoyle 1886: plate XII figs 1–7; shell plate XIII figs 1, 2; see Figs 58–59).
Robson (1932) considered both “Cirroteuthis” magna and “Cirroteuthis” hoylei to differ from Arctic Cirroteuthis muelleri. Guerra et al. (1998) redescribed “Cirrothauma” magna based on 2 new specimens from the Atlantic, but considered that Robson (1932) erred when describing “Cirroteuthis” hoylei, which should not have been attributed to anything other than genus (Guerra et al. 1998: p. 77).
O’Shea (1999) placed “Cirroteuthis” magna in Cirrothauma based on similarities in shell morphology and overall similarity to Cirrothauma murrayi. Collins & Villanueva (2006), also regarded Robson’s Valparaiso “Cirroteuthis” hoylei to be nomen dubium. While the shells of both “Cirroteuthis” magna and “Cirroteuthis” hoylei types are presumed lost (Jonathan Ablett, BMNH, pers. comm.), Hoyle’s excellent illustrations enable characterisation of this species, despite the poor condition of type material today. The “butterfly shaped” shells of specimens attributed to “Cirroteuthis” magna by Guerra et al. (1998) are more anteriorly elongated than shells of either “Cirroteuthis” magna or “Cirroteuthis” hoylei types, but they are more similar to them than they are to those of Cirroteuthis (s.s.). While both “Cirroteuthis” magna and “Cirroteuthis” hoylei are more similar to Cirrothauma murrayi (vs. Cirroteuthis) in shell form, arm length, lack of web nodules, and in having suckers that extend to the arm tips, molecular evidence indicates that they are best accommodated in a separate genus characterised (most notably, morphologically) by the possession of functional eyes and mid-arm suckers with distinct acetabular chambers on relatively short/absent stalks.
Sucker counts (to at least 53, and probably to 60) and the mid-arm sucker form of I. hoylei are similar to those of I. magna and differ from those of Cirroteuthis (s.s.): both Cirroteuthis muelleri and Cirroteuthis kirrilyae sp. nov. have fewer than 40 suckers per arm (Cirrothauma s.s. has ~36–60 (Chun 1913, Aldred et al. 1983)). For Cirroteuthis (e.g., Cirroteuthis kirrilyae sp. nov.) the mid-arm suckers are reduced, the acetabular chamber is absent, and the sucker is positioned on a fleshy peduncle. Mid-arm suckers of Cirrothauma murrayi are similar, and possibly even more reduced, and represented by small dishes atop large fleshy peduncles (Aldred et al. 1983: fig. 28; also Figs 50–51). Mid-arm suckers of I. magna and I. hoylei are similar, with well-formed and rounded barrel-like acetabulae that are loosely attached to the arm surface. Specimens attributed to Cirroteuthis magna by Guerra et al. (1998) were described with proximal suckers on thick/stout stalks, and mid arm suckers on narrow stalks – a description that is inconsistent with the I. hoylei holotype. Despite damage to the I. hoylei holotype, several intact arm tips lack web nodules.
A possible further specimen at the Field Museum of Natural History (Chicago, USA) (FMNH 309245) from the northeastern Pacific appears to be similar to the type of I. hoylei, and a CO1 sequence from this specimen is available (CO1 sequence ON367804.1, see Fig. 1); its placement reveals it to be distinct from I. magna. This specimen was only investigated from photographs, and its shell has not been dissected out but it is large (~67 mm ML), has arms ~2.5–3.0× ML, ~7 lamellae per gill demibranch (~14 per gill), and ~90 suckers per arm; suckers were divided into proximal-arm type suckers (suckers 1–14 compact, with bases almost touching, with suckers 10–12 largest (~1.5–2.0 mm ⌀)), followed by smaller and well-spaced mid-arm suckers (suckers 15–34 (⌀ ~50–60% larger proximal sucker ⌀, with ~3 sucker ⌀ spaces between adjacent mid-arm suckers)), and distal-arm suckers (from suckers 34–90+, compact with bases almost touching, larger than mid-arm suckers at ~1.3–1.5× mid-arm sucker ⌀, largest between suckers ~35 and 50); cirri are short (~1 sucker ⌀) until sucker 14 or 15, at which point their length rapidly increases (4× mid-arm sucker ⌀ by sucker 18, and up to 6–7× mid-arm sucker ⌀ by sucker 25 or 26); cirrus length reduces between distal-arm suckers to ~1× sucker ⌀ between suckers ~34–40. While these three sucker types are similar to those of I. hoylei, especially in the mid-arm region where they lack any kind of stalk/pedicle, we cannot discount the possibility that it represents a separate taxon (because of its excessively high distal-arm sucker counts).
KEY TO GENERA AND SPECIES OF SUPERFAMILY CIRROTEUTHOIDEA
To better facilitate the ongoing study of cirrates, a key is outlined below of known representatives in the superfamily Cirroteuthoidea (Froekenia Hoyle, 1904 is provisionally treated as a synonym of Stauroteuthis (Verhoeff 2023a)).
1a. Secondary webbing present, cirri generally elongate (> 3–10× sucker ⌀) . . . . . . . . . . . . (2)
1b. Secondary webbing absent, cirri generally short to moderate in length (< 3× sucker ⌀) . . . . . . . . . . . . . . Superfamily Opisthoteuthoidea
2a. Internal shell broadly U- or V-shaped, gills with 8 lamellae . . . . . . . . . . . . . . . . . . . . . . . . . . . Stauroteuthidae (genus Stauroteuthis) (3)
2b. Internal shell “saddle” or “butterfly” shaped, gills with ≥ 10 lamellae . . . . . . Cirroteuthidae (5)
3a. Cirri terminating between suckers 18–26 on dorsal arms, suckers small or large, shell with or without prominent lateral ‘shoulders’ . . . . . (4)
3b. Cirri terminating between suckers 28–30 on dorsal arms; suckers small (⌀ < 2.5 mm), shell with prominent lateral shoulders . . . . . . . . . . . . . . . . . . . . . . . . . Stauroteuthis kengrahami
4a. With suckers 1–3 largest (to 2.2 mm ⌀) in females, and 9–22 enlarged in males (~5–6 mm ⌀); shell with prominent ‘shoulders’ Stauroteuthis syrtensis
4b. With abruptly enlarged (conical) suckers ~5–23 in both sexes (largest 6–12, 5–9 mm ⌀), shell without ‘shoulders’ . . . . . Stauroteuthis gilchristi
5a. Shell “saddle” shaped (longer than wide), arms 2–3× ML, web nodules present . . Cirroteuthis(6)
5b. Shell “butterfly” shaped (wider than long), arms 3–5× ML, web nodules absent . . . . . . . . . (7)
6a. With 28–34 suckers on dorsal arms [Arctic Ocean] . . . . . . . . . . . . Cirroteuthis muelleri
6b. With 36–39 suckers on dorsal arms [Pacific and Indian Oceans] . . . . . . . . Cirroteuthis kirrilyae
7a. Eyes vestigial (no lens/opening), suckers vestigial, lacking acetabulum & atop very long fleshy stalks . . . Cirrothauma murrayi [species complex]
7b. Eyes well-developed (normal), suckers with distinct acetabulum, on at most short and thin stalks mid-arm . . . . . . . . . . . . . Inopinoteuthis (8)
8a. Mid-arm suckers on small stalks/peduncles . . . . . . . . . . . . . . . . . . . Inopinoteuthis magna
8b. Mid-arm suckers not on stalks/peduncles . . . . . . . . . . . . . . . . . . . . Inopinoteuthis hoylei
DISCUSSION
Cirroteuthis kirrilyae sp. nov. may be more widely distributed throughout the Indian and Pacific Ocean Basins, because ROV observations (Figs 40–43) have captured imagery of specimens consistent with what we now describe for this taxon across the northeastern, central, and southeastern Pacific. Small animals (outstretched webbing diameter ~30 cm) attributable to Cirroteuthis have been reported from Philippine Trench (~50 km east of Samar Island’s southern tip, 6,212−6,224 m) (Jamieson & Vecchione 2022) and the Clipperton–Clarion fracture zone (central Pacific, 4,750–4,800 m) (Vecchione 2017). As recognised Cirroteuthis muelleri is restricted to the Arctic; records of Cirroteuthis cf. muelleri from the abyssal northeastern Atlantic from ~55−45°N (from the Porcupine Abyssal Plain and Porcupine Seabight, 2,567–4,854 m) (Collins et al. 2001b) may be comparable, and a specimen from the mouth of Porcupine Seabight (Discovery Stn 9756) is comparable in sucker counts to Arctic material (Table 4). Molecular evidence suggests that Cirroteuthis from the central north Atlantic (Charlie Gibbs Fracture Zone, Sutton et al. 2010) are genetically distinct from Arctic Cirroteuthis muelleri, as are sequences from an unidentified Cirroteuthis supposedly collected off Antarctica, while Cirroteuthis kirrilyae sp. nov. (from northwestern Australia) was genetically distinct from all other Cirroteuthis species, with which it still forms a well-supported genus clade (Fig. 1).
While Pardo-Gandarillas et al. (2021) concluded that I. hoylei could “only be identified as a cirrate in general …” we believe otherwise. Although few described characters or their states differentiate this species from I. magna, we cannot discount the possibility that any differences are not mere preservation artefacts, or related to ontogeny or sex. Nevertheless, we refrain from advocating the synonymy of these two taxa, or treating I. hoylei as a nomen dubium because molecular evidence indicates that two species do exist; a pair of near-identical I. magna sequences from the North Atlantic and southern Indian Ocean (off Kerguelen) are well-separated from a third sequence from the northeastern Pacific (from specimen FMNH 309245, which is morphologically comparable to I. hoylei). The Kerguelen sequence is from a specimen collected close to the type locality of I. magna, which if correctly identified, extends the distribution of I. magna from the Indian Ocean to the Atlantic; animals from at least the eastern and northeastern Pacific belong to a different species, for which one potentially available name is I. hoylei.
Treating I. magna and I. hoylei in a genus separate from Cirrothauma and Cirroteuthis seems to be the best way to explain morphological and molecular data, though more molecular data are needed to confirm relationships between these taxa to others in the family. The recognition of Inopinoteuthis enables the diagnoses of Cirrothauma and Cirroteuthis to be more refined than when I. magna and/or I. hoylei are forced into one of them.
Molecular evidence (Fig. 1) indicates that Cirrothauma murrayi is a species complex, with one species occurring in both the Pacific and North Atlantic oceans, and a second species co-occurring at least in the North Atlantic. Aldred et al. (1983) noticed morphological differences among their North Atlantic Cirrothauma murrayi in terms of arm length and sucker counts that were not obviously attributable to specimen damage; their specimens ‘A–C’ (ML 130–220 mm) had arm lengths ~3× ML with sucker counts in “upper 30s”, whereas a smaller specimen ‘D’ (ML 105 mm) had arm length ~4× ML and sucker count ~60. Presumably these two forms match the two species indicated in sequences (but which sequence matches what morphotype is unknown); regardless, the type specimen of Cirrothauma murrayi had an ASC of 36 on all arms (ML 40 mm) (Chun 1911–1913: pp. 136–140), so the form with ~60 suckers probably awaits description. Until the systematics of these deep-sea cirrates are better known it is premature to refer to anything as new, for an appropriate name might currently be buried within the synonymy of another taxon.
Future efforts to acquire specimens Cirroteuthis, Cirrothauma, and Inopinoteuthis and their sequence data across their geographic range, using more nuclear and mitochondrial genes, is required to better understand relationships between these enigmatic creatures. Despite being some of the earliest of cirrates to be described, the cirroteuthids offer much potential for further study.