INTRODUCTION
The phylum Mollusca is the second most diverse animal group on Earth (Bouchet et al. 2016). A global estimation of freshwater gastropods and bivalves counts about 7,000 species (MolluscaBase 2024a). Despite their ecological importance (Zieritz et al. 2022), there is a considerable gap in our knowledge of species even in relatively well-studied geographic areas (Lopes-Lima et al. 2021). For example, several new species and genera have been described in the last twenty years (e.g. Sands et al. 2020, Miller et al. 2023) in the Mediterranean region, a well-known hotspot of freshwater biodiversity (Strong et al. 2008, Georgopoulou et al. 2016a), demonstrating that our knowledge of these molluscs in southern Europe is far from complete. Similarly, new taxa have been added to the list of Greek freshwater molluscs (e.g. Radea et al. 2013, 2016, 2021, Glöer & Hirschfelder 2020, Hofman et al. 2021, Glöer & Fischer 2022). Up until now, updated species lists are known only for certain regions or islands (e.g. Seidl 2001, Georgopoulou et al. 2016b) or water bodies (e.g. Albrecht et al. 2009, 2012, Georgopoulou et al. 2016a), while several parts of the country remain largely unexplored.
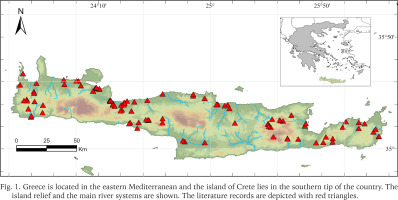
The island of Crete lies in the southernmost tip of Greece and Europe, and it is the fifth largest island in the Mediterranean Sea. Crete is home to a great number of endemic animals and plants species (e.g. Kougioumoutzis et al. 2020, Bolanakis et al. 2024). The island’s natural history is shaped by a combination of factors including the long isolation from any other land during the last five million years (Meulenkamp 1985), the mosaicity of the land that creates a plethora of habitats and the continuous presence of humans at least during the last ca. 8,000 years (Rackham 2008). The island has several rivers, mostly with intermittent flow, as well as, natural lakes, spring-lakes, springs, wells and dams. Recently, WWF Greece presented a catalogue of 200 natural and artificial wetlands throughout the island of Crete and adjacent islets (https://www.ygrotopio.gr/), among which, 69 are protected under a Presidential Decree (PD 229/19.06.2012).
The first record of a freshwater mollusc from Crete, currently accepted as Melanopsis buccinoidea (Olivier, 1801), was provided by Olivier (1801, 1804) in his two-volume work entitled “Voyage dans l’ Empire Othoman, l’ Égypte et la Perse… la République”. Later, additions to the island’s freshwater molluscan fauna were provided by Raulin (1869). Martens (1889) provided the first checklist of freshwater molluscs of Crete. During the 20th century, a historical review of Cretan molluscs, including terrestrial and freshwater species was published by Seidl (1978). The most up to date catalogue of Greek (including Cretan) freshwater molluscs with an extensive bibliographic review was assembled by Bank (2006). The European checklist of terrestrial and freshwater gastropods (Bank & Neubert 2017) also included the Cretan species, but the list did not seem to be exhaustive, as some species mentioned by Bank (2006) were omitted. According to Bank (2006) and Bank & Neubert (2017) 15 and 12 species are known to be present in Crete, respectively. Recently, Glöer & Hirschfelder (2019) made a list of the freshwater gastropods of Crete, 19 species in total, without giving other information than the species names.
Recent research effort has focused on the study of species lineages living in Crete, combining morphological and molecular analyses. For example, the study of the genus Pseudamnicola Paulucci, 1878 suggested the presence of at least two separate lineages on the island, that can only be identified by molecular analyses as their shell and genital characteristics are similar (Lampri et al. 2022). On the other hand, the genus Bythinella Moquin-Tandon, 1856, is represented in Crete by at least five species (groups) with distinct morphological and anatomical features (Lampri et al. 2022). Another species that has been studied from the island of Crete is Heleobia maltzani (Westerlund, 1886), now accepted as Eupaludestrina maltzani (Westerlund, 1886) (MolluscaBase 2024b). E. maltzani was originally described from Crete (Westerlund 1886) and was considered an endemic species, however Szarowska et al. (2014) showed that the species did not differ anatomically or genetically from its congeneric relatives and thus, concluded that it was probably a common widespread species in the Mediterranean and European semi-saline waters. Finally, other taxa, that have been surveyed in the east Mediterranean but not with a particular focus on the island of Crete, include the genera Theodoxus Montfort, 1810 (Sands et al. 2020) and Melanopsis Férussac, 1807 (Falniowski et al. 2020).
The aim of this work is to compile and present an up-to-date annotated checklist of names of the freshwater gastropods and bivalves of Crete based on an extensive bibliographic research effort. In addition, a map showing all identified locations bearing freshwater molluscs from Crete is provided. The study will serve as a reference for future research efforts focusing on Cretan freshwater molluscs.
MATERIAL AND METHODS
The annotated checklist is a result of an exhaustive literature review of publications, including books, research articles, conference abstracts and internet sources. Taxa records retrieved from the above listed sources were matched, when possible, with geographic coordinates of the location given in the source. Taxa records which could not be assigned geographic coordinates were maintained for the checklist but were not depicted in the accompanying distribution map (Fig. 1). Such cases refer to (mostly 19th century) publications, where the author(s) mentioned general names of places e.g. “Crete” and therefore it was not possible to associate the location with a set of geographic coordinates. Nomenclature in the annotated checklist follows MolluscaBase (2024a).
RESULTS
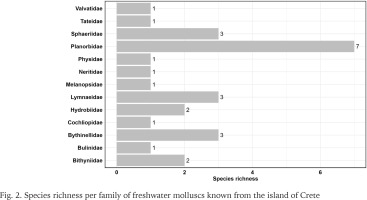
The checklist contains 308 taxa records deriving from 49 publications spanning in a period of 223 years, i.e. 1801 to 2024. It was possible to match 204 taxa records with geographic coordinates from the 308 (Fig. 1, Supplementary Table S1). In total, 27 accepted species and two subspecies were identified. The 27 species belonged to 13 families and 22 genera. The most species-rich family was Planorbidae Rafinesque, 1815 with seven species, followed by Lymnaeidae Rafinesque, 1815, Bythinellidae Locard, 1893 and Sphaeriidae Deshayes, 1855 (1820) with three species each (Fig. 2). Another 11 taxa were found but were excluded from the checklist: i) three taxa, Planorbis rotundatus Poiret, 1801, Ancylus gibbosus Bourguignat, 1853 and Peregriana labiata (Rossmässler, 1835) are classified as taxa inquirenda, ii) the known distributions of six taxa, Theodoxus anatolicus (Récluz, 1841), Theodoxus baeticus (Lamarck, 1822), Ancylus capuloides Jan in Porro, 1838, Bythinella sp., Bythinella viridis (Poiret, 1801) and Adriohydrobia gagatinella (Küster, 1852) do not include Crete, iii) it was not possible to trace any information for the species Pisidium creticum Cless., and thus its current status remains uncertain, and iv) the species Theodoxus peloponensis (Récluz, 1841) is a dubious species and its known distribution does not include Crete.
ANNOTATED CHECKLIST
Phylum Mollusca
Class Gastropoda Cuvier, 1795
Subclass Neritimorpha Koken, 1896
Order Cycloneritida Frýda, 1998
Superfamily Neritoidea Rafinesque, 1815
Family Neritidae Rafinesque, 1815
Subfamily Neritininae Poey, 1852
Genus Theodoxus Montfort, 1810
1. Theodoxus fluviatilis (Linnaeus, 1758)
Neritina Heldreichi – Martens 1879: 225, pl. 22, fig. 9, 10
Neritina (Theodoxus) heldreichi var. graeca – Westerlund 1886: 152
Neritina (Theodoxus) heldreichi var. graeca – Kobelt 1899: 9, pl. 213, fig. 1337
Theodoxus saulcyi – Schütt 1986: 285, fig. 12–14
Theodoxus saulcyi – Frank 1988: 82
Theodoxus (Theodoxus) saulcyi – Bank 2006: 52
Theodoxus (Theodoxus) saulcyi – Bank & Neubert 2017: 3
Theodoxus (Theodoxus) saulcyi – Glöer & Hirschfelder 2019: 22, table 1
Theodoxus saulcyi – Sands et al. 2019: Appendix S1, table S1.1
Theodoxus saulcyi – Sands et al. 2020: table S1, fig. 10L
Remarks: Although several names are known regarding Theodoxus from Crete, recent molecular analyses showed that only the Theodoxus fluviatilis clade lives in the island (Bunje & Lindberg 2007, Sands et al. 2019). T. fluviatilis is distributed across Europe to northern Africa and Asia Minor and the European part of Russia (Sands et al. 2020 and references therein). In Crete the species has been found in springs, rivers, and lakes (Schütt 1986, Glöer & Hirschfelder 2019).
Subclass Caenogastropoda Cox, 1960
Superfamily Cerithioidea Fleming, 1822
Family Melanopsidae Adams et Adams, 1854
Genus Melanopsis Férussac, 1807
2. Melanopsis buccinoidea (Olivier, 1801)
Melania buccinoïdeaOlivier1801: 297, pl. 17, fig. 8
Melanopsis buccinoidea (Melania) – Férussac & Férussac 1807: 710–771
Melanopsis praerosa (Buccinum) – Raulin 1869: 988
Melanopsis praerosa – Westerlund & Blanc 1879: 143
Melanopsis praerosa – Hesse 1883: 80
Melanopsis praerosa = buccinoidea – Martens 1889: 189, 222, 232, table II, III
Melanopsis buccinoidea – Sturany 1904: 112
Melanopsis praerosa-buccinoidea – Fuchs & Käufel 1934: 72
Melanopsis (Melanopsis) praemorsa buccinoidea – Fuchs & Käufel 1936: 542–543
Melanopsis buccinoidea – Seidl 1978: 173
Melanopsis buccinoidea – Tillier & Mordan 1983: 157
Melanopsis praemorsa buccinoidea – Frank 1988: 82
Melanopsis buccinoidea [= ferussaci Roth, 1839] – Glaubrecht 1993: 46, fig. 1, pl. 1, fig. 12
Melanopsis buccinoidea – Bank 2006: 53
Melanopsis buccinoidea – Bank & Neubert 2017: 10
Melanopsis buccinoidea – Glöer & Hirschfelder 2019: 22, table 1
Remarks: Melanopsis buccinoidea was described by Olivier (1801) as Melania buccinoïdea. However, Olivier (1801) did not specify the type locality, simply mentioning that the species lived in Chios Island, almost all the island of the Archipelago (Aegean Sea), in Crete and Syria. Raulin (1869) considered M. buccinoidea as a synonym of Melanopsis praerosa. Similarly, Westerlund & Blanc (1879) synonymized M. buccinoidea with M. praerosa without referring to the work of Raulin (1869). Later, Hesse (1883) reproduced the list of species from Raulin (1869) in order to make Raulin’s work accessible to more malacologists and therefore listed again the species as “M. praerosa”. Martens (1889) referred to the species thrice. First the species was mentioned as Melanopsis praerosa (L.) from Chania in page 189 (Martens 1889). Then, in table II in page 222 the species was listed as “Melanopsis praerosa (L.) = buccinoidea Olivier” and then again in table III in page 232 as “Melanopsis praerosa L. (buccinoidea Olivier)”. Considering the above, I assume that Martens (1889) treated the “Melanopsis praerosa L.” of Crete a synonym to the species Melanopsis buccinoidea. In the 20th century, Fuchs & Käufel (1934, 1936) mentioned that M. buccinoidea is predominant in the Aegean islands including Crete. Seidl (1978) considered that the records from Raulin (1869), Westerlund & Blanc (1879) and Martens (1889) belong to M. buccinoidea. Glaubrecht (1993) stated that the species from Crete is M. buccinoidea, but erroneously referred to it as M. praemorsa in pl. 1, fig. 12 in page 93. Neiber & Glaubrecht (2019) recognised a distinct lineage of Melanopsis in the eastern Mediterranean and the Near to Middle East, which includes the species M. buccinoidea. Furthermore, the distribution of the species M. praemorsa (Linnaeus, 1758) seems to be restricted to Spain and north-western Africa (Neiber & Glaubrecht 2019, Falniowski et al. 2020). At the same time, a most recent research study showed that M. buccinoidea is restricted to northern Aegean islands (Falniowski et al. 2020). However, neither Neiber & Glaubrecht (2019) nor Falniowski et al. (2020) provide any information on the melanopsids of Crete. Considering all the above, I assigned all records of M. praemorsa from Crete to the name M. buccinoidea. However, the taxonomic and phylogenetic study of fresh material from Crete is considered imperative in order to reveal the true identity of the Cretan melanopsids. In Crete the species is known from springs, streams and rivers.
Order Littorinimorpha Golikov et Starobogatov, 1975
Superfamily Truncatelloidea Gray, 1840
Family Bithyniidae Gray, 1857
Genus Bithynia Leach in Abel, 1818
3. Bithynia candiota (Westerlund, 1886)
Bythinia badiella var. candiota Westerlund 1886: 22
Bithynia badiella var. candiota – Seidl 1978: 173
Bithynia candiota – Frank 1988: 83
Bithynia (Codiella?) candiota – Bank 2006: 53
Bithynia candiota – Glöer & Maassen 2009: 42, figs 21–24
Bithynia candiota – Bank & Neubert 2017: 12
Bithynia candiota – Glöer & Hirschfelder 2019: 22, table 1
Remarks: The species was described by Westerlund (1886) as “B. badiella var. candiota”. The type locality provided by the author was “Candia” which is the Venetian name for Crete or Heraklion (the largest city of Crete). B. candiota is endemic to Crete, known to live in the rivers of the island (Glöer & Maassen 2009, Glöer & Hirschfelder 2019).
4. Bithynia cretensis Glöer et Maassen, 2009
Bithynia cretensis Glöer & Maassen 2009: 42–43, figs 31–36
Bithynia cretensis – Bank & Neubert 2017: 12
Bithynia cretensis – Glöer & Hirschfelder 2019: 22, table 1
Remarks: B. cretensis is a Cretan endemic species (Glöer & Maassen 2009). The species is known from a stream in Lasithi in eastern Crete (Glöer & Maassen 2009).
Family Hydrobiidae Stimpson, 1865
Genus Pseudamnicola Paulucci, 1878
5. Pseudamnicola brachia (Westerlund, 1886)
Paludinella (Pseudamnicola) brachia Westerlund 1886: 76–77
Amnicola exotica – Martens 1889: 232, table 2
Bythinella (Bythinella) brachia – Jaeckel 1967: 95
Pseudamnicola (Pseudamnicola) exotica – Pieper & Willmann 1978: 121
Bythinella brachia – Pieper & Willmann 1978: 129
Amnicola exotica – Seidl 1978: 173
Pseudamnicola brachia – Schütt 1980: 132–133, fig. 29, Abb. 4, Taf. 10
Pseudamnicola (Pseudamnicola) brachia – Bank 2006: 53
Pseudamnicola brachia – Szarowska et al. 2014: 2488–2489, figs 2–4
Pseudamnicola brachia – Glöer & Hirschfelder 2015: 111
Pseudamnicola brachia – Szarowska et al. 2016b: fig. 7 A–D, fig. 10 L–O,
Pseudamnicola brachia – Bank & Neubert 2017: 16
Pseudamnicola brachia – Glöer & Hirschfelder 2019: 12–13, figs 1, 3
Remarks: The name A. exotica Martens, 1889 was considered a nomen nudum by Bank (2006) and a synonym of P. brachia. P. brachia is an endemic species of Crete and has been reported from springs and streams (Schütt 1980).
6. Pseudamnicola occultus Glöer et Hirschfelder, 2019
Pseudamnicola occulta Glöer & Hirschfelder 2019: 13–15, figs 4–10
Pseudamnicola sp. – Lampri et al. 2022: 810, table 1
Pseudamnicola occultus – Lampri et al. 2024: 850, table 1
Remarks: The type locality of P. occultus is a spring in Chania (Glöer & Hirschfelder 2019). Moreover, Glöer & Hirschfelder (2019) suggested that the specimens figured in Szarowska et al. (2016b) corresponded with P. occultus. Similarly, they suggested it was probable that Schütt (1980) mistakenly presented P. occultus as P. brachia. Finally, Lampri et al. (2024) referred to the Pseudamnicola Group I of Lampri et al. (2022) as P. occultus. P. occultus is a Cretan endemic species.
Family Cochliopidae Tryon, 1866
Genus Eupaludestrina Mabille, 1877
7. Eupaludestrina maltzani (Westerlund, 1886)
Paludinella (Hydrobia) maltzani Westerlund 1886: 37
Hydrobia maltzani – Martens 1889: 232, table III
Hydrobia (Hydrobia) maltzani – Pieper & Willmann 1978: 120
Paludinella (Hydrobia) maltzani – Seidl 1978: 173
Semisalsa maltzani – Schütt 1980: 117, fig. 3, Taf. 9
Heleobia (Semisalsa) maltzani – Bank 2006: 54
Heleobia maltzani – Szarowska et al. 2014: 2488–2489, figs 2–4
Heleobia (Eupaludestrina) maltzani – Bank & Neubert 2017: 15
Heleobia (Eupaludestrina) maltzani – Glöer & Hirschfelder 2019: 22, table 1
Remarks: Bank (2006) and Bank & Neubert (2017) considered that E. maltzani is endemic to Crete, but according to Szarowska et al. (2014) it is probably a common European brackish water species.
Family Bythinellidae Locard, 1893
Genus Bythinella Moquin-Tandon, 1856
8. Bythinella cretensis Schütt, 1980
Bythinella (Bythinella) badiella candiota – Jaeckel 1967: 95
Bythinella badiella – Pieper & Willmann 1978: 129
Bythinella cretensis – Schütt 1980: 130–131, fig. 27, Abb. 3, Taf. 10
Bythinella cretensis – Bank 2006: 56
Bythinella cretensis – Szarowska et al. 2014: 2488–2489
Bythinella cretensis – Bank & Neubert 2017: 36
Bythinella cretensis – Glöer & Hirschfelder 2019: 15–16, figs 14–18
Bythinella cretensis – Glöer & Porfyris 2022: 32, figs 3.8, 4.8
Remarks: Schütt (1980) and Glöer & Porfyris (2022) considered that the Hydrobia (Bythinella) sp. mentioned by Martens (1889) represented a specimen of B. cretensis. Nevertheless, these statements cannot be validated until the material of Martens has been checked. The species B. cretensis is known from springs, rivers and streams of Crete (Schütt 1980, Glöer & Hirschfelder 2019).
9. Bythinella rethymnonensis Glöer et Hirschfelder, 2020
Bythinella magdalenae Glöer & Hirschfelder 2019: 16–17, figs 20–24
Bythinella rethymnonensis Glöer & Hirschfelder 2020: 7
Bythinella rethymnonensis Glöer & Porfyris 2022: 34–35, figs 3.29, 4.29
Remarks: It is a Cretan endemic that lives in springs (Glöer & Hirschfelder2019).
10. Bythinella sitiensis Glöer et Hirschfelder, 2019
Bythinella sitiensis Glöer & Hirschfelder 2019: 17–18, figs 25–28
Bythinella sitiensis – Glöer & Porfyris 2022: 35, figs 3.32, 4.32
Remarks: It is an endemic that lives in springs of eastern Crete (Glöer & Hirschfelder 2019).
Family Tateidae Thiele, 1925
Genus Potamopyrgus Stimpson, 1865
11. Potamopyrgus antipodarum (Gray, 1843)
Potamopyrgus antipodarum – Lampri et al. 2017: 45
Remarks: This is the first record of the alien species P. antipodarum from Crete, and the second from Greece overall (see Radea et al. 2008).
Subclass Heterobranchia
Superfamily Valvatoidea Gray, 1840
Family Valvatidae Gray, 1840
Genus Valvata Müller, 1773
12. Valvata kournasi Glöer et Hirschfelder, 2019
Valvata kournasiGlöer & Hirschfelder 2019: 17–18, figs 40–42
Remarks: This is a recently described species, known only from its type locality, Lake Kournas, in western Crete (Glöer & Hirschfelder 2019). Glöer & Hirschfelder (2019) erroneously mentioned that figs 37–42 correspond to V. kournasi. The species depicted in figs 37–39 is V. macrostoma.
Superorder Hygrophila
Superfamily Lymnaeoidea Rafinesque, 1815
Family Lymnaeidae Rafinesque, 1815
Genus Galba Schrank, 1803
13. Galba truncatula (Müller, 1774)
Lymnaea truncatula (Buccinum) – Raulin 1869: 987
Lymnaea truncatula – Hesse 1883: 79
Limnaea truncatula – Martens 1889: 189, 232, table 3
Lymnaea (Galba) truncatula – Fuchs & Käufel 1936: 543
Lymnaea truncatula – Seidl 1978: 173
Galba (Galba) truncatula – Bank 2006: 57
Remarks: There are no recent sightings of G. truncatula from Crete.
Genus Radix Montfort, 1810
14. Radix auricularia (Linnaeus, 1758)
Radix auricularia – Lampri et al. 2017: 45
Remarks: A single mention of the species presence in Crete was provided in a conference abstract by Lampri et al. (2017). No information on the species habitat preferences or the water body type that the species was found in the island of Crete was provided.
Genus Ampullaceana Servain, 1882
15. Ampullaceana lagotis (Schrank, 1803)
Ampullaceana lagotis – Aksenova et al. 2018: 4, Supplementary Dataset 1
Remarks: A specimen belonging to the species A. lagotis from Lake Kournas in Chania was analysed for molecular analyses by Aksenova et al. (2018).
Family Physidae Fitzinger, 1833
Genus Physella Haldeman, 1842
16. Physella acuta (Draparnaud, 1805)
Physa capillata Gassies in Raulin 1869: 987
Physa capillata – Gassies 1870: 306–307
Physa capillata – Hesse 1883: 79
Physa capillata – Martens 1889: 232, table III
Physa capillata – Seidl 1978: 173
Haitia acuta – Bank 2006: 58
Remarks: P. capillata was described by Gassies in Raulin (1869). The species description was provided again by Gassies (1870) in “Journal de Conchyliologie”. Here I followed Bank (2006) who considered P. capillata a synonym of P. acuta.
Family Bulinidae Fischer et Crosse, 1880
Genus Bulinus Müller, 1781
17. Bulinus truncatus (Audouin, 1827)
Physa brocchii – Westerlund & Blanc 1879: 126
Physa contorta var. brochii – Seidl 1978: 173
Bulinus truncatus – Schütt 1987: 243–244
17a. Bulinus truncatus contortus (Michaud, 1829)
Physa contorta – Martens 1889: 232, table III
Physa contorta – Seidl 1978: 173
17b. Bulinus truncatus rivularis (Philippi, 1836)
Bulinus (Isidora) truncatus rivularis – Bank 2006: 58
Bulinus (Isidora) truncatus rivularis – Glöer & Hirschfelder 2019: 22, table 1
Bulinus (Isidora) truncatus rivularis – Bank & Neubert 2017: 42
Remarks: Schütt (1987) discussed the ways of introduction of B. truncatus to the island of Crete and mentioned that his work was the first to report the species from Crete. However, this statement was incorrect. The species B. truncatus (Audouin, 1827) had been previously reported as “Physa brocchii Ehrenb.” from Crete by Westerlund & Blanc (1879), more than a century before Schütt (1987). Furthermore, Bank (2006) and Bank & Neubert (2017) considered only the subspecies B. truncatus rivularis to live in Crete. However, the subspecies B. truncatus contortus had been previously reported by Martens (1889) as well. The identity of the (sub)species of Bulinus living in Crete and the means of introduction require further investigation.
Family Planorbidae Rafinesque, 1815
Genus Planorbis Müller, 1773
18. Planorbis atticus Bourguignat, 1852
Planorbis atticus – Raulin 1869: 987
Planorbis Atticus – Hesse 1883: 80
Planorbis atticus – Meier-Brook 1976: 110
Planorbis atticus – Seidl 1978: 174
Planorbis planorbis (?) atticus – Frank 1987: 114
Planorbis (Planorbis) atticus – Bank 2006: 58
Planorbis (Planorbis) atticus – Glöer & Hirschfelder 2019: 22, table 1
Remarks: Glöer & Hirschfelder (2015) mentioned that Meier-Brook (1976) possibly misidentified a specimen of Planorbis cretensis Glöer et Hirschfelder, 2015 from Crete as P. atticus. Since, this information cannot be validated, I considered that the specimen of Meier-Brook (1976) belonged to P. atticus. Frank (1987) listed the “small subspecies atticus (Bourguignat, 1852)” mentioned by Seidl (1978) under Planorbis planorbis (Linnaeus, 1758). Since, no other record of P. planorbis from Crete exists, and previous publications (Bank 2006, Bank & Neubert 2017) did not mention the species from Crete, I assume that the species that Frank (1987) referred to is P. atticus. P. atticus is endemic to Greece, its range includes central Greece, the Ionian islands, Andros island in the Cyclades and the island of Crete (Glöer & Pešić 2010, Georgopoulou et al. 2016b). In Crete the species is known from a stream and the outflow of lake Kournas.
19. Planorbis cretensis Glöer et Hirschfelder, 2015
Planorbis cretensisGlöer & Hirschfelder 2015: 110–111, fig. 1
Planorbis (Planorbis) cretensis – Bank & Neubert 2017: 42
Planorbis (Planorbis) cretensis – Glöer & Hirschfelder 2019: 22, table 1
Remarks: The information on the species distribution is limited. More research on the relationship with its congeneric species is required. P. cretensis is a Cretan endemic species known from a spring and the outflow of Lake Kournas (Glöer & Hirschfelder 2015, 2019).
Genus Anisus Studer, 1820
20. Anisus leucostoma (Millet, 1813)
Anisus (Anisus) leucostoma – Bank 2006: 59
Anisus (Anisus) leucostoma – Bank & Neubert 2017: 43
Anisus (Anisus) leucostoma – Glöer & Hirschfelder 2019: 22, table 1
Remarks: Although listed by Bank (2006), Bank & Neubert (2017) and subsequently by Glöer & Hirschfelder (2019), no literature record before Bank (2006) could be found and according to Glöer (2019) the species is not present in Greece or Crete. Nevertheless, as I considered the list of Bank (2006) a reliable source, I retained A. leucostoma in the species list. Further investigation is suggested in order to validate the species presence in Crete.
Genus Gyraulus Charpentier, 1837
21. Gyraulus parvus (Say, 1817)
Planorbis glaber – Martens 1889: 232, table 3
Planorbis glaber – Seidl 1978: 173
Remarks: P. glaber is a junior subjective synonym of Gyraulus laevis (Alder, 1838), formerly considered a European native species. Recently, G. laevis became a synonym of G. parvus, a North American species, under the light of molecular analyses (see Lorencová et al. 2021). “G. laevis” and G. parvus belong to the same species-level clade in the phylogenetic tree, but the former constitutes a European race with a North American origin and the latter a North American race (Lorencová et al. 2021). Lorencová et al. (2021) hypothesized that “G. laevis” is an early introduction of the North American race into Europe 500 years ago or earlier, while the North American race is a recent introduction of G. parvus into Europe. Glöer (2024) rejected this synonymy, basing his opinion on differences between the two species anatomy and ecology. Further research is needed to clarify the possible distinctness between G. laevis and G. parvus, as well as, the species status in Crete.
Genus Armiger Hartmann, 1843
22. Armiger crista (Linnaeus, 1758)
Armiger crista – Maassen 1992: 94
Gyraulus (Armiger) crista – Bank 2006: 59
Remarks: The species was not mentioned from Crete either by Bank & Neubert (2017) or Glöer & Hirschfelder (2019). In Crete A. crista is known to live in rivers (Maassen 1992).
Genus Segmentina Fleming, 1818
23. Segmentina nitida (Müller, 1774)
Planorbis nitidus – Martens 1889: 189, 232, table 3
Planorbis nitidus – Seidl 1978: 173
Segmentina nitida – Bank 2006: 59
Segmentina nitida – Bank & Neubert 2017: 45
Segmentina nitida – Glöer & Hirschfelder 2019: 22, table 1
Remarks: S. nitida was first mentioned from western Crete by Martens (1889). Subsequent references of the species from Crete were probably based on the species list given by Martens (1889).
24. Ancylus fluviatilis Müller, 1774
Ancylus fluviatilis – Schütt 1982: 521
Ancylus fluviatilis – Bank 2006: 59
Ancylus fluviatilis – Glöer & Hirschfelder 2019: 22, table 1
Remarks: Glöer & Hirschfelder (2019) erroneously put the author’s name in brackets [Ancylus fluviatilis (Müller, 1774)]. The species was not mentioned by Bank & Neubert (2017). In Crete A. fluviatilis has been reported from springs and streams (Schütt 1982, Glöer & Hirschfelder 2019).
Class Bivalvia Linnaeus, 1758
Subclass Autobranchia Grobben, 1894
Order Sphaeriida Lemer, Bieler et Giribet, 2019
Superfamily Sphaerioidea Deshayes, 1855 (1820)
Family Sphaeriidae Deshayes, 1855 (1820)
Subfamily Sphaeriinae Deshayes, 1855 (1820)
Genus Sphaerium Scopoli, 1777
25. Sphaerium lacustre (Müller, 1774)
Sphaerium lacustre – Martens 1889: 232, table III
Sphaerium lacustre – Seidl 1978: 173
Remarks: S. lacustre has not been reported from Crete since Martens (1889), as Seidl (1978) only reproduced Martens’ list. The species was not mentioned from Crete by Bank (2006).
Genus Euglesa Jenyns, 1832
26. Euglesa casertana (Poli, 1791)
Pisidium Casertanum (Cardium) – Raulin 1869: 983
Pisidium casertanum – Westerlund & Blanc 1879: 144
Pisidium Casertanum – Hesse 1883: 78
Pisidium fossarinum var. ovale – Martens 1889: 232, table III
Pisidium casertanum – Westerlund 1890: 27–28
Pisidium casertanum – Seidl 1978: 173
Pisidium fossarinum var. ovale – Seidl 1978: 173
Remarks: E. casertana has not been reported from Crete in recent years. I could not trace the name “Pisidium fossarinum var. ovale Cless.” in the literature or available online databases e.g. MolluscaBase (2024a) or MUSSELp (https://mussel-project.uwsp.edu/) except from the publications of Martens (1889) and Seidl (1978). Nevertheless, I considered it as a synonym of E. casertana as the name Pisidium fossarinum Clessin is a synonym of E. casertana.
Genus Odhneripisidium Kuiper, 1962
27. Odhneripisidium annandalei (Prashad, 1925)
Pisidium vincentianum – van Regteren 1957: 132, table V
Pisidium annandalei – Kuiper 1962b: 7
Pisidium annandalei – Kuiper 1981: 81
Pisidium (Odhneripisidium) annandalei – Bank 2006: 61
Remarks: The name P. vincentianum is a junior subjective synonym of Odhneripisidium stewarti (Preston 1909) (MolluscaBase 2024c). However, I followed Kuiper (1981) who considered that P. vincentianum mentioned by van Regteren (1957) is P. annandalei.
DOUBTFUL TAXA
Theodoxus anatolicus (Récluz, 1841)
Theodoxus (Neritaea) anatolicus – Bank & Neubert 2017: 3
Theodoxus (Neritaea) anatolicus – Glöer & Hirschfelder 2019: 22, table 1
Remarks: There are strong indications that T. anatolicus is restricted to south-western Anatolia (Sands et al. 2019, 2020) and thus its presence in Crete is doubtful. I do not consider that the species lives in Crete. Nevertheless, more research focused on the populations of Theodoxus from the island of Crete is needed in order assess its true diversity.
Theodoxus baeticus (Lamarck, 1822)
Neritina Boetica – Raulin 1869: 988
Neritina Boetica – Hesse 1883: 80
Neritina boeotica – Seidl 1978: 174
Remarks: T. baeticus is widespread throughout the Mediterranean (see Sands et al. 2020 and references therein), but no recent study verifies its presence in Crete. The single record of the species in Crete was from Raulin (1869). Subsequent authors, i.e. Hesse (1883) and Seidl (1978), reproduced Raulin’s species list without adding new field data. In addition, the study of the phylogeny of the genus Theodoxus revealed that the specimens from Crete belonged to T. fluviatilis (Sands et al. 2020). Thus, its presence remains to be confirmed (or rejected) by new studies.
Theodoxus peloponensis (Récluz, 1841)
Neritina peloponnesiaca – Martens 1889: 232, table III
Neritina peloponnesiaca – Seidl 1978: 173
Remarks: Although T. peloponensis is an accepted taxon name in MolluscaBase (2024d), there were suggestions that the name was a synonym of T. baeticus (Bank 2006) and recently its status was considered dubious (Sands et al. 2020).
Planorbis rotundatus Poiret, 1801 (taxon inquirendum)
Planorbis rotundatus – Raulin 1869: 987
Planorbis rotundatus – Hesse 1883: 80
Planorbis rotundatus – Martens 1889: 189, 232, table III
Planorbis rotundatus – Seidl 1978: 173
Remarks: The species P. rotundatus is currently classified as a taxon inquirendum (MolluscaBase 2021).
Ancylus capuloides Jan in Porro, 1838
Ancylus capuloides – Martens 1889: 189
Ancylus capuloides – Seidl 1978: 173
Remarks: Schütt (1982) mentioned that the species A. capuloides was known from Crete. Following this, Frank (1987) suggested that A. capuloides mentioned by Seidl (1978) referred to the species Ancylus fluviatilis f. gibbosus Bourguignat, 1853 (see below). However, the combination is superseded (MolluscaBase 2024e) and the accepted name A. gibbosus Bourguignat, 1853 is considered as a taxon inquirendum (MolluscaBase 2024f). In addition, Frank (1987) mistakenly mentions as citation “Seidl 1968”, the correct being “Seidl 1978”. At present, A. capuloides is known from Italy (MolluscaBase 2024g). Field studies and a combined effort of morphological and genetic approach is required in order to understand the taxonomic position of Ancylus of Crete.
Ancylus gibbosus Bourguignat, 1853 (taxon inquirendum)
Ancylus fluviatilis f. gibbosus – Frank 1987: 114
Remarks: The species A. gibbosus is currently classified as a taxon inquirendum (MolluscaBase 2024f). Further investigation is required to disentangle the taxon’s status.
Bythinella sp.
Bythinia similis (Cyclostoma) – Raulin 1869: 987
Bythinia similis (?) – Hesse 1883: 80
Bithynia similis – Seidl 1978: 174
Remarks: Raulin (1869) probably refers to the species Cyclostoma simile Draparnaud, 1805 which is currently a synonym of Mercuria similis (Draparnaud, 1805). However, it is unlikely that the record of “Bythinia similis (Cyclostoma)” by Raulin (1869) refers to M. similis since the species is known from the Mediterranean coast of France and Spain and the island of Mallorca (Glöer et al. 2015) and the genus Mercuria is restricted to the western Mediterranean, north-western Africa and Atlantic coastal regions (see Glöer et al. 2015, Boeters & Falkner 2017, Miller et al. 2023 for details).
Bythinella viridis (Poiret, 1801)
Bythinia viridis (Bulimus) var. inflata ? – Raulin 1869: 987
Bythinia viridis (?) var. conflata ? – Hesse 1883: 80
Bithynia viridis: Seidl1978: 174
Remarks: Bythinella viridis is endemic to France (Prie & Cucherat 2021), and therefore the species presence on the island is doubtful.
Adriohydrobia gagatinella (Küster, 1852)
Hydrobia kutschigi – Fuchs & Käufel 1934: 72
Remarks: Currently, A. gagatinella is known from Montenegro, Croatia and possibly Albania (Wilke & Falniowski 2001, Bank & Neubert 2017), and there are no records of the species from Greece. Fuchs & Käufel (1934) note that the Cretan form differs from the type specimen of H. kutschigi, currently known as A. gagatinella. The Cretan form is slimmer, with rapidly increasing whorls and a smaller aperture (Fuchs & Käufel 1934). In the same publication it also mentioned that A. J. Wagner, who collected the specimen, confirms the species but considers it a separate form. Acknowledging the above information, I assume that the authors misidentified the specimen from Crete as A. gagatinella and therefore the species presence in Crete is highly unlikely.
Peregriana labiata (Rossmässler, 1835) (taxon inquirendum)
Radix labiata – Glöer & Hirschfelder 2019: 22, table 1
Remarks: A single mention of the species from the outflow of Lake Kournas in western Crete is provided by Glöer & Hirschfelder (2019). It is possible that the specimens found by Glöer & Hirschfelder (2019) belong to A. lagotis which was also found in Lake Kournas by Aksenova et al. (2018). It is currently considered as taxon inquirendum (MolluscaBase 2024h) and new field data will assist the discovery of which lymnaeid species live(s) in Crete.
Pisidium creticum Clessin (uncertain taxon)
Pisidium creticum – Martens 1889: 232, table III
Pisidium creticum – Seidl 1978: 173
Remarks: There is no record of the name “Pisidium creticum Cless.” in any publication or relevant online database e.g. MolluscaBase (2024a) or MUSSELp (https://mussel-project.uwsp.edu/) except from the publications of Martens (1889) and Seidl (1978). Also, I was not able to retrieve the publication that contains the taxon’s original description. The taxon’s current taxonomic status is uncertain and further research is required in order to reveal its true identity.
DISCUSSION – GENERAL REMARKS
The current annotated checklist is the most recent and up-to-date contribution to our knowledge of the freshwater molluscs of Crete. The in-depth study of the available literature revealed that at least 24 species and two subspecies of gastropods, and three bivalve species have been documented by previous authors to live in Crete. The results show that the freshwater gastropods and bivalves of Crete have been sporadically studied in the past, and only recently have there been efforts to research the island’s freshwater gastropods (e.g. Szarowska et al. 2014, Glöer & Hirschfelder 2015, 2019, Lampri et al. 2022). The geographic distribution of localities indicates that field sampling was focused on certain water bodies, e.g. Lake Kournas and Almyros river in Western Crete, while certain areas such as the mountain massifs of the island have been overlooked (see Fig. 1).
The number of freshwater molluscs known from Crete has increased compared to the most recent lists provided by previous authors (Bank 2006, Bank & Neubert 2017, Glöer & Hirschfelder 2019). This was expected as new species were described in the last decade (e.g. Glöer & Hirschfelder 2015, 2019). Of the 27 species, eight are endemic to Crete, i.e. Bithynia candiota, B. cretensis, Pseudamnicola brachia, P. occultus, Bythinella cretensis, B. sitiensis, Valvata kournasi and Planorbis cretensis. In the case of E. maltzani, recent molecular analyses showed that the species is not endemic to Crete as previously thought (Szarowska et al. 2014). The islands paleogeographic history and landscape complexity, justify the high number of (endemic) species when compared to other Greek islands such as Andros (Georgopoulou et al. 2016b), Kos (Avrithis & Fischer 2022), Lesvos (Bank 1988) and Samos (Bank & Maassen 1998). Nevertheless, more research is necessary in order to make safe comparisons since our knowledge of freshwater molluscs from the Greek islands is incomplete.
The alien species P. antipodarum was recorded for the first time from Crete and in general from insular Greece (Lampri et al. 2017). The presence of this alien species, its current population status and distribution in Crete need to be investigated. Another presumably alien species for Europe, namely G. parvus, has been documented from the island under the name “P. glaber” (Martens 1889, Seidl 1978). At the moment two races, i.e. “G. laevis” and G. parvus, are placed under the name G. parvus (Lorencová et al. 2021). However, without the study of fresh material, it is impossible to know which race lives and when it arrived in Crete. I urge for a modern investigation of the species from the island of Crete in order to understand its history and assist to the untangling of its taxonomic status.
More new species are expected to be described from Crete as indicated by recent integrative analyses (see Lampri et al. 2022). The genus Bythinella has been investigated by two independent research groups in Crete (Szarowska et al. 2016a, 2016b, Lampri et al. 2022). Cumulative results of these previous studies indicate the presence of at least 5 Bythinella spp. in the island, but so far, they have refrained from naming the species (see Szarowska et al. 2016a, Lampri et al. 2022). The species B. cretensis, B. rethymnonensis and B. sitiensis are included within the aforementioned identified species. I have refrained from assigning the records from Szarowska et al. (2016a) and Lampri et al. (2022) under a species name, nevertheless their records were included in Figure 1.
Similarly, the phylogeny of the genus Pseudamnicola has been analyzed by Szarowska et al. (2016b) and Lampri et al. (2022, 2024). The paper of Szarowska et al. (2016b) focused on the Aegean islands and included a single location from Crete, while Lampri et al. (2022) provided a thorough study of the genus exclusively from Crete. The latest comprehensive phylogeny of the genus Pseudamnicola across the Mediterranean, included species from Crete. Molecular analyses suggest that possibly two species of Pseudamnicola live in Crete, one is attributed to P. occultus (Lampri et al. 2024), but the other one cannot be safely attributed to P. brachia (Lampri et al. 2022, Lampri et al. 2024) the other species of Pseudamnicola already known from the island. Furthermore, Lampri et al. (2024) suggests, that the species Pseudamnicola chia (von Martens, 1889) may also live in Crete, however this requires validation from morphological and anatomical studies.
Our knowledge of the freshwater molluscan fauna of Crete is by far complete. Field studies focusing on both previously searched localities as well as new ones are highly desired. The study of the species ecological preferences, their phylogenetic relationships and biogeographical affinities will add to our understanding of the freshwater fauna of the island. This work aims to become a starting point for modern research efforts on the freshwater molluscan taxonomy and ecology, as well as, contribute to conservation planning of the Cretan fresh waters.