INTRODUCTION
The genus Gyraulus Charpentier, 1837 is the most species-rich group of freshwater gastropods belonging to the Planorbidae family, with about 200 valid species (MolluscaBase 2021a), of which nearly thirty occur in the Western Palaearctic (Glöer 2019). Among them, the nominal species Planorbis rossmaessleri Auerswald, 1852 (in Schmidt 1852) remains particularly poorly studied. Indeed, after Geyer (1927), this species was considered in the literature as a “variety” or subspecies of Gyraulus gredleri (Gredler, 1859), which was subsequently treated by Meier-Brook (1964) as a junior synonym of Gyraulus acronicus (A. Férussac, 1807). Based on anatomical characters of the reproductive organs, Meier-Brook (1964, 1983) recognised P. rossmaessleri as a distinct and valid species within the genus Gyraulus, whereas Starobogatov (1967) placed it in the genus Choanomphalus Gerstfeldt, 1859. According to Vinarski et al. (2006), the generic assignment of P. rossmaessleri is still controversial, although MolluscaBase recommends treating it within the genus Gyraulus.
Its overall geographical range is also poorly known. The species was considered to be strictly European (see for example Meier-Brook 1983), but data provided by Vinarski et al. (2006) obtained from various museum collections show that it is also widespread throughout most of Siberia. The geographic range of the species thus appears to cover a large part of the northern Palearctic, from the Upper Rhine Basin at its westernmost limit (Richling & Groh 2014, Umbrecht & Bichain 2018) to eastern Siberia at the Lena Basin on its easternmost limit, but also extends beyond the Arctic Circle on the Yamal Peninsula (Vinarski et al. 2006). More specifically, Gyraulus rossmaessleri has been documented in north-eastern France (Umbrecht & Bichain 2018), Germany (Meier-Brook 1983, Richling & Groh 2014, Glöer 2019), Austria (Falkner 1995), Romania (Glöer & Sîrbu 2005, Sîrbu et al. 2011), Czech Republic (Juřičková et al. 2001, Beran 2002, 2005, Beran & Horsák 2011), Slovakia (Lučivjanská & Šteffek 1991, Čejka 2000, Čejka et al. 2005, Čacany & Čejka 2009), Latvia (Pilāte 2013), Poland (Piechocki 1979, Czyż et al. 2016), Hungary (Richnovszky & Pintér 1979, Fehér et al. 2006), Lithuania (Schlesch 1942, Seddon 2011), Russia and Ukraine (Uvayeva & Gural 2008, Kantor et al. 2009). Paleontological data suggest a more westerly distribution over the Pleistocene, with occurrences in the Netherlands, United Kingdom, Denmark, and northern France (e.g. Meijer 1988, 2010, Limondin-Lozouet 2001, Preece 2003, Parfitt et al. 2010).
The species is mainly documented as living in various temporary wetlands such as alder groves, overgrown swamps, small temporary pools, ditches and canals located in floodplain forests or grasslands (Meier-Brook 1983, Falkner et al. 2001, Glöer 2019), apparently in unpolluted water (Obrdlik et al. 1996). In addition, Beran & Horsák (2011) described an atypical habitat in the Czech Republic in small wetlands around spring areas and also in small slow-flowing rivulets. These authors also showed that the species was not found in habitats under strong anthropogenic pressure, such as industrial or domestic water pollution, but only in protected natural areas. This also seems to be the case in France (Umbrecht & Bichain 2018).
Although the ecology, biology, and life cycle of G. rossmaessleri are poorly documented (Piechocki 1979), field observations suggest that the species survives prolonged habitat desiccation for several weeks or even months. The physiological or behavioural mechanisms involved have never been studied, but individuals could survive periods of drought into the wettest microhabitats and slowing or stopping their growth, as indicated by the scars left on their shell (Beran & Horsák 2011, Umbrecht & Bichain 2018, Bichain 2024).
In France, the species is only known in Alsace (Gargominy et al. 2011, Bichain et al. 2019), an administrative region that partially covers the Upper Rhine Valley near the border with Germany (Fig. 1). The first records are attributed to Geissert (1960), under the name of Gyraulus gredleri, and refer to flooded meadows north of Strasbourg (Figs 1–2). Some of this Alsatian material was studied by Meier-Brook (1961, 1964, 1983) as part of his revision of the genus Gyraulus, and in particular its separation into G. rossmaessleri and G. acronicus. Most of these habitats have been destroyed or severely degraded (Geissert 1988) and the species has not been recorded in Alsace for over 30 years. However, Umbrecht & Bichain (2018) discovered the species in similar habitats in central Alsace in the Illwald Regional Nature Reserve. Subsequently, the IUCN categorisation of the species was updated to Endangered [EN] at national (UICN Comité Français, OFB & MNHN 2021) and regional (ODONAT Grand Est 2023) level.
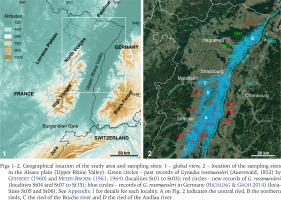
Figs 1–2
Geographical location of the study area and sampling sites: 1 – global view, 2 – location of the sampling sites in the Alsace plain (Upper Rhine Valley). Green circles – past records of Gyraulus rossmaessleri (Auerswald, 1852) by Geissert (1960) and Meier-Brook (1961, 1964) (localities St01 to St03); red circles – new records of G. rossmaessleri (localities St04 and St07 to St15); blue circles – records of G. rossmaessleri in Germany (Richling & Groh 2014) (localities St05 and St06). See Appendix 1 for details for each locality. A on Fig. 2 indicates the central ried, B the northern rieds, C the ried of the Bruche river and D the ried of the Andlau river
Since Umbrecht & Bichain’s (2018) publication, other populations and localities of G. rossmaessleri have been recorded in this region (Bichain et al. 2023) but have not been precisely described. Given the ongoing anthropogenic pressures on wetlands, it seems crucial to provide a comprehensive overview of these records and a regional baseline for future research, in order to reduce the major shortfalls and handicaps affecting the knowledge and conservation of this species (Lopes-Lima et al. 2021).
The main objectives of this paper are therefore (1) to describe the habitats where populations have been observed in northeastern France, (2) to provide diagnostic tools for the unambiguous identification of the species, (3) to review the literature in order to better understand its past distribution on its western border and what is already known about its biology and ecology, and finally (4) to propose an update of its threat status in the French IUCN Red List.
MATERIAL AND METHODS
STUDY AREA
The Alsace Ried consists of a mosaic of floodplain meadows and forests, criss-crossed by a dense network of rivers, springs and phreatic streams (Heuacker et al. 2015). It extends over a surface of ca. 990 km2 (Sell et al. 1998), mainly in the northern half of the Alsace Plain in north-eastern France on the border with Germany (Fig. 1). Altitudes range from 120 m to 180 m in the north and south of the plain respectively. There are several distinct rieds (Fig. 2), the main ones being (i) the central ried, also called [in French] Ried Ello-rhénan, (ii) the northern rieds (rieds du Nord), (iii) the ried of the Andlau river (ried d’Andlau) and (iv) the ried of the Bruche river (ried de la Bruche). The central ried (zone A on Fig. 2) is the largest, covering an area of ca. 170 km2 and extending 50 km from the south of Sélestat to the south of Strasbourg (Bernhard et al. 1992). It is bordered to the west by the Ill river and to the east by the Rhine. The northern rieds (zone B on Fig. 2) are located in a vast wetland area stretching some twenty kilometres north of Strasbourg. This area is bounded to the west by the eastern edge of the Mio-Pliocene terrace of the Haguenau forest, and to the east by the Rhine. Finally, to the west of the Ill river, lies the Andlau ried (zone D on Fig. 2), bordered by the sub-Vosgian foothills, and the Bruche ried (zone C on Fig. 2) which correspond to a vast wet depression between Molsheim and Strasbourg.
The flooding of the rieds can have three origins: (i) the overflowing of rivers; (ii) the emergence of the Rhine water table in micro-depressions (e.g. ditches, old channels) and/or the overflowing of phreatic rivers; (iii) the temporary effect of localised heavy rainfall, during summer storms or rapid snowmelt in the lowlands, which can saturate the soil and retain water in scattered puddles of varying size. Typically, the rieds are more or less permanently flooded during the periods of rain and/or snowmelt from autumn (November) to spring (March–April).
The meadow ecosystems of the rieds consist mainly of purple moor-grass Molinia caerulea (L.) Moench, 1794 meadows, low marshes, sedge meadows and reed beds. The forests are mainly mixed riparian forests, with oak, elm and ash trees, and forest patches of alder and willow.
Almost all the rieds have been classified as Natural Zone of Ecological, Faunistic and Floristic Interest (ZNIEFF), i.e. natural areas inventoried for their remarkable ecological value. The ZNIEFFs have no restrictive regulatory value but complement the protected areas in order to guide land-use planning decisions and prevent artificialisation. Each ZNIEFF received a descriptive sheet (available at https://inpn.mnhn.fr) detailing the different habitats, surface areas and the checklist of inventoried species. These data were used to determine the total surface area of habitats potentially favourable to G. rossmaessleri, which was used to calculate both the maximum area of occurrence (EOO) and the maximum area of occupancy (AOO) required for the IUCN categorisation.
OCCURRENCE DATA AND SAMPLING METHODS
The occurrence data were acquired during malacological field inventories carried out in various floodplain meadows during the period 2018–2024 (Fig. 2, Appendix 1). These data are based on opportunistic sampling, i.e. without a quantitative protocol, by sampling and sieving litter in the dry season (generally from May to October), or with fine-mesh sieves (500 μm) in the wet season (generally from November to April). Sampling was carried out in habitats suitable for the species, mainly in natural areas managed by the Conservatory of the natural areas of Alsace (Conservatoire d’espaces naturels d’Alsace or CEN Alsace in the following text) and in the Illwald Regional Nature Reserve (Illwald RNR in the following text).
Data cited in the literature were included if the name of the sampling site (at least at the town level of precision) and of the observer, as well as the sampling date (at least the year) were clearly specified, and if the diagnostic characters used were explicitly provided and/or linked to one or more specimens in a private or public collection.
We consider a locality to potentially host a population capable of reproduction and dispersal when both live adults and juveniles have been observed. When possible, the EUNIS (2018) or Habitats Directive codes are given.
PALEONTOLOGICAL DATA
The critical literature review of Quaternary fossil occurrences of Gyraulus rossmaessleri was based on publications in English, French, German, Dutch and by accessing the Quaternary Molluscan DataBase (2024). The aim of this work was to provide a spatiotemporal view of its range, focusing on the western margins of its current distribution. The records of this taxon were examined for chronological and palaeoclimatic context by checking the type of dating of the site, which is mostly radiocarbon method for the Late Pleistocene and Holocene (Preece 2003, White et al. 2008, Meijer 2010). Chronological attribution of Early and Middle Pleistocene deposits is based on stratigraphic correlation and biostratigraphy (Meijer 1990, Puisségur 1978, Parfitt et al. 2010). Fossil occurrences without radiometric control have also been under consideration, but with a degree of caution. The sedimentary context and associated mollusc fauna have also been considered.
ANATOMY
For dissections, the animals were quickly scalded and then, to facilitate extraction of the animal’s soft body parts, the shells were first dissolved/fragilised with 10% HCl for approximately 5 minutes. The extracted tissues were immediately and thoroughly washed with water and fixed in 70% ethanol to both stop the effects of hydrochloric acid and to slightly harden the tissues. All dissections were performed under a stereomicroscope (Motic SMZ 171) using thin pointed forceps (0.06 mm). Reproductive systems were dissected out and then photographed using a digital camera (Tucsen 20 MP) mounted on a microscope (Nikon YS100). Line drawings were made by hand from these photographs. The terminology used here to describe the different parts of the reproductive system follows Meier-Brook (1983).
ABBREVIATIONS
Institutions and collection: CEN Alsace – Conservatoire d’espaces naturels d’Alsace; Illwald RNR – Illwald Regional Nature Reserve; MHNEC – Musée d’Histoire naturelle et d’Ethnographie, Colmar (France); MZS – Musée Zoologique de Strasbourg (France).
Anatomical description (text and figures): bc – bursa copulatrix, bcd – duct of the bursa copulatrix, ca – carrefour, dd – deferent duct, mrp – penis retractor muscle, ngl – nidamental gland, od – oviduct, pfg – female genital pore, pl – pilaster, pmg – male genital pore, pp – penis pore, prp – preputium, psh – penis sheath, pst – prostat gland, sa – sarcobelum, sod – spermoviduct, spd – sperm duct, st – stylet, vd – vas deferens, ut – uterus, vg – vagina, vl – velum, wp – penis wall.
RESULTS
Class Gastropoda Cuvier, 1795
Superorder Hygrophila Férussac, 1822
Family Planorbidae Rafinesque, 1815
Genus Gyraulus Charpentier, 1837
Gyraulus rossmaessleri (Auerswald, 1852)
Original combination. Planorbis rossmaessleri Auerswald, 1852 in Schmidt (1852: 179–183).
Synonyms. Anisus starobogotovi Frolova, 1984; Planorbis planoconcavus Westerlund, 1897 (Vinarski et al. 2006, MolluscaBase 2021b).
Previous (sub)generic assignments. Starobogatov (1967: 296) established the subgenus Lamorbis of the genus Choanomphalus Gerstfeld, 1859 with Gyraulus riparius (Westerlund, 1865) as the type species, and also included P. rossmaessleri. Meier-Brook (1983) recognises this subgenus, on the basis of the structure of the male reproductive apparatus, but assigns it to the genus Gyraulus as sister group to subgenus Armiger W. Hartmann, 1843. Furthermore, Prozorova & Starobogatov (1999) established the subgenus Pseudogyraulus of the genus Choanomphalus with Planorbis rossmaessleri Auerswald, 1852 as the type species.
According to Vinarski et al. (2006), since Starobogatov (1967), P. rossmaessleri has been treated by Russian and Ukrainian authors in the genus Choanomphalus, while Western European authors follow Meier-Brook (1983) in using the combination Gyraulus rossmaessleri with or without assignment to the Larmobis or Pseudogyraulus subgenera. Consequently, the species is still variously named in the literature with, for example, the combinations Lamorbis (Pseudogyraulus) rossmaessleri (e.g. Thorp et al. 2015, Vinarski & Kantor 2016), Choanomphalus (Pseudogyraulus) rossmaessleri (e.g. Vinarski & Khokhutkin 2013) or Pseudogyraulus rossmaessleri (e.g. Saito et al. 2018).
The planorbid molecular phylogeny approach of Albrecht et al. (2007) suggests that the genera Choanomphalus and Gyraulus form separate clades. However, the different molecular phylogenetic analyses using mitochondrial and/or nuclear sequences attributed to G. rossmaessleri do not allow to assign the species unambiguously within the genus Gyraulus (see for example von Oheimb et al. 2013, Sitnikova & Peretolchina 2018, Saito et al. 2018, 2023, Lorencová et al. 2021). The systematic position of G. rossmaessleri therefore remains unresolved, and we follow the recommendation of MolluscaBase, which places this species within the genus Gyraulus based on the current state of planorbid taxonomy.
Original description. Testa depressa, supra concava, subtus perspective umbilicata, anfractibus 4 celeriter accresscentibus, subteretibus, non carinatis, subtiliter transverse striatis, apertura transverse lunato-ovata, peristomate albolabiato. Alt. 11/2, diam. maj. 5, min. 4 millim.; apert.11/2 mill. alta, 2 lata (Schmidt 1852: 179). [English translation: shell depressed, concave above, umbilicated below, with 4 rapidly and regularly increasing turns, not keeled, finely transversely striated, transverse and oval-shaped aperture, peristome with white lip. Shell height: 1.5 mm, maximum shell diameter: 5 mm, minimum shell diameter: 4 mm, aperture: 1.5 mm high and 2 mm wide.]
Type locality. Habitat fossam limosal prope Lipsias (Schmidt 1852: 179). [English translation: lives in a silty ditch near Leipzig.]
Type material. unknown (Kantor et al. 2009 and not specifically searched for by us).
Material examined. Approximately 200 shells and/or live animals from 14 sites sampled between 2018 and 2024 (St04 to St15, see Appendix 1) were examined in this study. The material collected by Geissert and Meier-Brook (St01 to St04, see Appendix 1) was not examined. Detailed information on each sampled site is given in Appendix 1 and the location of the corresponding sites is mapped in Figs 1–2.
Habitats. In the study area, the species has been observed in 17 separate localities (Appendix 1), including 9 new ones since Umbrecht & Bichain (2018). In the northern rieds, empty shells and live specimens were collected in a flood depression occupied by sedge meadows and willow grove with ash (Durr & Thierry 2020 and this article, locality St4 on Fig. 2 and Appendix 1). This locality probably corresponds to the one mentioned by Geissert et al. (1985) and Geissert (1988), under the name of Rossteigwiese, which in the 1960s, must have been a damp or wet oligotrophic meadow, now in the process of natural closure. The suitable habitats for the species in the northern rieds area cited by Geissert (1960, 1961) and Meier-Brook (1961, 1964, 1983) (localities St1 to St3 on Fig. 2 and Appendix 1), formerly purple moor-grass meadows, were destroyed in the 1970s and 1980s by the development of urbanisation and/or agricultural activities (Geissert et al. 1985, Geissert 1988).
This species has also been sampled in two distinct localities in the ried of the Andlau river (localities St10 and St14 on Fig. 2 and Appendix 1). The corresponding habitats are flooded depressions with large sedges in hygrophilous Molinia caerulea meadows under the influence of overflow from adjacent rivers and/or rising groundwater table.
In the central ried, active juvenile and adult specimens were observed during the first floods of November-December 2023 at two sites mentioned by Umbrecht & Bichain (2018) within the boundaries of the Illwald RNR (locality St07 on Fig. 2 and Appendix 1, Figs 5–7), and in particular in a dense stand of Carex acuta L., 1753 (locality St07a on Fig. 2, Appendix 1 and Fig. 7) and in a small depression in a meso-hygrophilous hay meadow with water ragwort Jacobaea aquatica (Hill) G. Gaertn., B. Mey. & Scherb., 1801 invaded by large helophytes (locality St07e on Appendix 1 and Fig. 2). In the latter, 54 individuals were sampled, of which 88% were juveniles with a shell size of less than 2 mm. During this flood season, the species was also observed active north of the Illwald RNR, in the flooded depressions of large meso-hygrophilous meadows (locality St08 on Fig. 2 and Appendix 1, Fig. 3). In all cases, the microhabitats correspond to flooded ditches of medium depth (<50 cm) located at the edge of the tree cover. South of the Illwald RNR, but outside its administrative boundaries, live adults and juveniles were collected from a willow-invaded sedge meadow between an alder grove and a reed bed (locality St15 on Fig. 2 and Appendix 1).
Figs 3–8
Sampling sites of Gyraulus rossmaessleri (Auerswald, 1852) in the central ried: 3 – locality St08: flooded meadow in the Illwald Regional Nature Reserve, November 2023, 4 – locality St09: flooded hay meadow at Muttersholtz, December 2023, 5 – locality St07d: flooded hay meadow in the Illwald Regional Nature Reserve, August 2018, 6 – locality St07e: on the edge of the flooded hay meadow St07d, November 2023, 7 – locality St07a: on the edge of a sedge bed in the Illwald Regional Nature Reserve, November 2023, 8 – locality St11: on the edge of a sedge bed at Herbsheim, November 2023. See text and Appendix 1 for more detailed station descriptions

In the north of the central ried, specimens were observed active during the flood period and quiescent during the dry period in 4 other areas: (i) in flooded depressions in a large hay meadow (locality St09 on Fig. 2 and Appendix 1, Fig. 4); (ii) in a depression mainly occupied by a reed bed, at the edge of a meso-hygrophilous Molinia caerulea hay meadow (locality St11 on Fig. 2 and Appendix 1, Fig. 8); (iii) at the edge between a reed bed and a mesophilic to hygromesophilic hay meadow (locality St12 on Fig. 2 and Appendix 1) and (iv) in a sedge meadow dominated by Carex acuta, Carex disticha Huds., 1762, Carex vesicaria L., 1753, which also hosts species of high conservation value such as Lathyrus palustris L., 1753, Jacobaea paludosa (L.) G. Gaertn., B. Mey. & Scherb., 1801 and Carex buxbaumii (locality St13 on Fig. 2 and Appendix 1).
Based on these results, it appears that the habitats found in northern France where the species occurs correspond to damp or wet oligotrophic grasslands (EUNIS 2018: E3.5), in particular with Molinia caerulea (EUNIS 2018: E3.511; EU Habitats Directive: 6410), hygromesophilic hay meadows at low and medium altitudes (EUNIS 2018: E2.222; EU Habitats Directive: 6510), hygrophilic hay meadows flooded for long periods by rising groundwater (EUNIS 2018: E3.43; EU Habitats Directive: 6440), meadows with water ragwort (EUNIS 2018: E3.414) or marshy willow shrublands (EUNIS 2018: F9.21). The plant communities correspond to stands of large sedges (EUNIS 2018: D5.21), including Carex buxbaumii (EUNIS 2018: D5.21A) or reed beds, usually without open water (EUNIS 2018: D5.11). Some of these habitats are of Community interest and are listed in Annex I of the EU Habitats Directive (codes: 6410, 6440, 6510). In these (macro)habitats, the species seems to prefer ditches, small channels, or depressions at the edge between open habitats (meadows) and dense plant communities (groves of trees or shrubs, reed beds or sedge meadows). The (micro)habitats occupied by the species are located in the lowest parts of the rieds and usually represent small patches no larger than a few dozen square metres within the meadows.
At all these localities, the species was observed either during the dry period with quiescent individuals (localities St4, St07a, St07b, St07c, St07d, St07e, St10, St13 and St14 on Fig. 2 and Appendix 1) and/or with active individuals during the flooding period (localities St07a, St07d, St07e, St08, St09, St11, St12 and St15 on Fig. 2 and Appendix 1). The presence of juveniles at all these stations suggests the presence of well-established population(s) potentially capable of dispersal.
Paleontological data. The planorbid Gyraulus rossmaessleri is commonly found in several Pleistocene and Holocene deposits (Table 1) in the heart of its current distribution sensu Glöer (2019). Fossil occurrences are located in Russia from Early to Late Holocene (White et al. 2008, Danukalova et al. 2011, Makarchuk et al. 2020, Yamskikh et al. 2022), in Late Pleistocene sequence of Poland (Alexandrowicz & Alexandrowicz 2010), in a Holocene site of Lithuania (Sanko et al. 2010), in Pleistocene undated loess profile of Czech Republic (Ložek 2001) or in Late Pleistocene and Late-glacial sequence in Germany (Geissert 1968, Mania et al. 1993). According to the palaeomalacological literature, the Pleistocene distribution of G. rossmaessleri shows a wider and more westerly range in areas where it is now considered to be extinct. In the United Kingdom and northern France, G. rossmaessleri occurred in Middle and Late Pleistocene sequences (Mazenot et al. 1963, Geissert et al. 1976, Puisségur 1978, Limondin-Lozouet 2001, Preece 2003, Parfitt et al. 2010), whereas Netherlands has the older Early Pleistocene record (Meijer 1990) and occurrences during the Late Pleistocene (Meijer 2010). In addition, this species has recently been found in a Late Glacial core level in Denmark (Bennike et al. 2019).
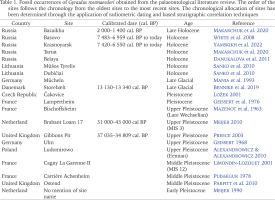
Table 1
Fossil occurrences of Gyraulus rossmaessleri obtained from the palaeontological literature review. The order of the sites follows the chronology from the oldest sites to the most recent sites. The chronological allocation of sites has been determined through the application of radiometric dating and based stratigraphic correlation techniques
Molluscan associated species are typical of cold tolerant fauna [e.g. Columella columella (G. von Martens, 1830), Vallonia tenuilabris (A. Braun, 1843), Trochulus cf. hispidus (Linnaeus, 1758), Succinella oblonga (Draparnaud, 1801)] commonly found in glacial and peri-glacial deposits (Ložek 1990). All of the fossil occurrences outside its modern range mentioned above are dated to Early, Middle, and Late Pleistocene glacial periods. Local palaeo-environmental reconstruction deduced from sediment and bioproxy analyses suggest cold and wet conditions in most of these sites. However, there are some exceptions, such as the existence of G. rossmaessleri in (i) Holocene deposits in Lithuania and Russia, where it is really common, and (ii) during an Eemian interglacial in Poland.
Chronoclimatic context of its past distribution and associated molluscan fauna suggest that G. rossmaessleri is a glacial relict species with a more fragmented distribution today than during the Quaternary period.
DIAGNOSTIC CHARACTERS
Shell morphology: Small to medium-sized shell, generally no larger than 7 mm in width and 1.3–1.5 mm in height, 4–4.5 rounded whorls; never angular or keeled (Figs 9 & 11–13). The last whorl slightly enlarged. Aperture oval shaped with a characteristic internal thickened white lip (Fig. 9). Teleoconch surface with very fine reticulated micro-sculpture and protoconch with about ten regularly spaced longitudinal striae (Fig. 13). Periostracum reddish brown. Shells may have up to 6 more or less pronounced scars, indicating interruptions in growth during dry periods (Figs 9 & 11–13). The first scar may be located just behind the protoconch (Fig. 13). During the periods of hydric distress, the animal withdraws deep into its shell and produces a convex lens-like epiphragm, horny coloured and relatively thick (Fig. 10).
Figs 9–13
Specimens of Gyraulus rossmaessleri (Auerswald, 1852) from the central ried: 9 – shell of a specimen sampled at locality St14, 10 – epiphragm (top: external view, bottom: internal view) of a quiescent specimen sampled at locality St07a, 11 – empty shells and shells with quiescent specimens (indicated by a red arrow) sampled at locality St07a during summer (August 2018), 12 – live specimen sampled at locality St08 during winter (November 2023) (red arrows indicate scars on the shell), 13 – shell of a juvenile specimen (the red arrow at the top indicates the second scar and the first at the end of the protoconch). Scale bars 1 mm (9), 500 μm (10), 5 mm (11), 2.5 mm (12), 250 μm (13)

Reproductive system (Figs 14–18): Distal spermoviduct long and very slender. Bursa copulatrix elongate club shaped or tapering. Bursa duct wide, not narrower than the vagina. Sperm duct wide. Prostate gland with low to moderate number of diverticula (8–21 after Meier-Brook 1983) closely spaced and regular in form (Fig. 14). Penis sheath extremely short and narrow scarcely set off from the vas deferens. Preputium considerably wider and longer than the penis sheath. Retractor muscle inserts on the terminal part of the penis sheath just before the emergence of the preputium (Figs 14–15). Penis sheath terminated by a sarcobelum (sensu Hubendick & Rees 1955; papilla sensu Baker 1945), a cone-shaped tissue with a hole through which the stylet passes during copulation. Preputium with usually one or two muscular pilasters (Figs 16 & 18), which correspond to invagination of the inner epithelium (Meier-Brook 1964, 1983). Presence of a muscular ring (or velum sensu Hubendick & Rees 1955, or diaphragm sensu Meier-Brook 1983) in the proximal part of the preputium (Figs 16 & 18).
Fig. 14
Reproductive system of Gyraulus rossmaessleri (Auerswald, 1852) from specimen sampled in the central ried (locality St11) [Drawing by J.-M. Bichain] (left: schematic view, right: line drawings after dissection). See text for abbreviations. Scale bar 1.5 mm
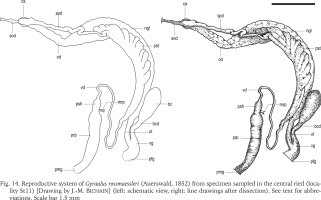
Figs 15–18
Male part of the reproductive system of Gyraulus rossmaessleri (Auerswald, 1852) from specimens sampled in the central ried (locality St11): 15 – view of the penis sheath, 16 – view of the transition between the penis sheath and preputium, 17 – stylet, 18 – schematic view of the transition between the penis sheath and preputium. See text for abbreviations. Scale bars 100 μm (15–16, 18), 25 μm (17)
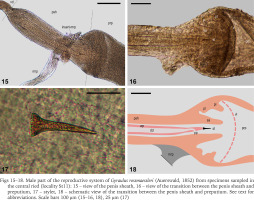
Penis tiny and slender with tapering tip. Penial pore (Figs 16 & 18), i.e. the deferens duct orifice, located where tapering begins and before the stylet. Stylet thin, conical shaped, with a length of c.a. 40–47 μm and a maximum diameter of c.a. 9 μm (Fig. 17). Its proximal part is enlarged, bowl-shaped, with fine longitudinal striations and an opening of around 7–8 μm (Soldatenko & Sitnikova 2009). Terminal part of the stylet thin and with no opening. According to Soldatenko & Sitnikova (2009), its function is probably not sperm conduction but only stimulation.
IUCN STATUS UPDATE IN FRANCE
Gyraulus rossmaessleri is currently listed in the French IUCN Red List (UICN Comité Français, OFB & MNHN 2021) as Endangered [EN B1ab(iii,v)+B2ab(iii,v)]. However, the new data presented here do not challenge this categorisation but need to be explained and slightly updated.
To minimise the effects of under-sampling, the maximum area of occurrence (EOO, B1 criterion) is estimated to be a maximum of 1,000 km2 (<5,000 km2), i.e. the total area over which the rieds extend (Heuacker et al. 2015). The maximum area of occupancy (AOO, B2 criterion) is estimated to be a maximum of about 300 km2 (<500 km2), i.e. the total area of habitats potentially suitable for the species within the EOO, estimated from the ZNIEFF sheets.
We estimate that the current and future impacts of intensive agriculture and global warming allow applications of options a (severely fragmented), b (continuing decline) and c (extreme fluctuation) (IUCN 2022). Intensive agriculture, mainly maize growing on a regional scale, not only considerably reduces the surface area of natural habitats but also has an impact on the quality of surface and groundwater through intensive irrigation, pollution by pesticides, diffuse nitrate and by other pollutants such as chloride or Volatile Organic Compounds (Bernhard et al. 1992, Rinaudo et al. 2005, Graveline et al. 2014, Kreins et al. 2015, Kempf & Glaser 2020). The predicted effects of global warming are to increase frequency and duration of droughts and, consequently, the critical quiescence period for species. Furthermore, a comparison with Pleistocene fossil distribution data in France indicates that (i) the range of this species has diminished over time, and (ii) the population has undergone a significant decline.
Although Gyraulus rossmaessleri has naturally dispersed populations with strong demographic fluctuations, we argue that these anthropogenic factors significantly worsen not only fragmentation (option a) but also continuing decline (option b) observed, estimated, inferred or projected in (i) extent of occurrence; (ii) area of occupancy; (iii) area, extent and/or quality of habitat; (iv) number of locations or subpopulations; (v) number of mature individuals as well as extreme fluctuations (option c) in (i) extent of occurrence; (ii) area of occupancy; (iii) number of locations or subpopulations; (iv) number of mature individuals.
Consequently, strict application of the IUCN criteria (IUCN 2022) would lead to the category Endangered [EN B1ab(i, ii, iii, iv, v)c(i, ii, iii, iv)+B2ab(i, ii, iii, iv, v)c(i, ii, iii, iv)] at the national scale.
DISCUSSION
Our results show that Gyraulus rossmaessleri has a wider distribution than expected (Umbrecht & Bichain 2018) in the French part of the Upper Rhine Valley, which represents its extreme western limit of distribution. The species has been recorded at 17 sites, most of them in the central ried and especially in the Illwald RNR. All the habitats occupied by the species match those described in the literature (Meier-Brook 1983, Falkner et al. 2001, Beran & Horsák 2011, Glöer 2019), i.e. diverse plant communities such as sedge meadows, phragmites reed beds or willow groves associated with floodplain meadows, some of which are of Community interest. They are all characterised by a more or less continuous period of water accumulation, lasting about 6–7 months from October to March–April, followed by a complete drying of the soil surface, also more or less continuous, from May to September. Although the regional range of G. rossmaessleri is estimated to be around 1,000 km2, the total area of presumed suitable habitats is probably much less than 300 km2. However, it is difficult to estimate the latter more precisely in the absence of regional cartography of the plant communities concerned. Nevertheless, we observe that the habitats where G. rossmaessleri occurs consist of small areas of a few tens or hundreds of square metres, isolated from each other mainly in areas of intensive cultivation. The only exception is the Illwald RNR, which comprises almost 19 km2 of natural environments, including flood meadows in a good state of conservation, where the species has been observed at 7 distinct sites.
Our study does not allow us to quantify a possible historical reduction in micro-distribution of the species in this region, although the destruction of some sites has already been observed (Geissert et al. 1985, Geissert 1988). However, the decline and fragmentation of these habitats throughout the Upper Rhine Valley is well documented (see for an overview Trémolières et al. 1998). Indeed, the rectification and canalisation of the Rhine in the 19th (1817–1876) and 20th (until 1977) centuries led to a drastic lowering of the water table, resulting in a considerable reduction in the total area of wet meadows, as well as in the amplitude and extent of flooding (Thierion et al. 2012). For example, the total area flooded by Rhine overflows was reduced by 85% after its canalisation (Trémolières et al. 1998). In addition, the conversion of wet meadows to arable land, especially since the 1960s, has had a significant impact on the number of floodplain meadows, which has decreased by about 64%, while the area of cultivated land has been multiplied by 30 (Clandillon & Defraipont 2001).
The Illwald RNR is an interesting case because, despite a large area of contiguous flood meadows, the species is mainly observed active in small patches of flooded old channels or depressions. In summer, in the damp litter of these depressions, or under dense plant cover, it is possible to find shells still containing the living but inactive animal, withdrawn deeply into the shell. Individuals can become active in a few hours after being placed in water (J.-M. Bichain, personal observation made under artificial conditions). Conversely, during the coldest periods of winter, the ried water may be completely frozen, although the deepest pools (> 20–70 cm) are likely to remain unfrozen. Both dry and probably cold periods slow or stop growth, leaving more or less pronounced scars on the shells, up to 6 in some cases. First scars may be located close to the protochonch, indicating that harsh conditions may occur shortly after hatching. These elements suggest that habitats suitable for G. rossmaessleri must provide a mosaic of microhabitats, more or less deep and sheltered from full sunlight, to escape not only frost in winter but also complete drought in summer. In addition, soil depressions are obviously both the first and the last places to be flooded (Stubbington & Datry 2013) and among the wettest micro-habitats in summer.
According to Strachan et al. (2015) and to Ansart & Vernon (2003), behavioural responses allow freshwater invertebrates to actively escape exposure to air or low temperatures by migrating to refuges that provide sufficient moisture or protection from freezing. This means that environmental dryness or cold does not lead necessarily to desiccation or freezing of organisms, which would involve more complex, often combined, physiological responses. Desiccation tolerance is defined as the ability to dry to equilibrium with air (<0.1 g H2O g−1) and then, to regain normal function after rehydration (Alpert 2005, Rebecchi et al. 2007), while cold hardiness covers a range of biochemical strategies that sustain life at subzero body temperatures (Storey & Storey 2004). In order to limit, resist or avoid desiccation or freezing, some species, particularly freshwater invertebrates or ectotherm vertebrates, have developed resistant life stages, alternative life cycles, rapid growth and/or complex metabolic mechanisms. Frost is also another form of desiccation, and adaptations to low temperature and drought are likely to use some similar adaptive strategies (Ansart & Vernon 2003).
However, behaviour or physiological adaptations of freshwater gastropods to extreme cold and/or desiccation are poorly studied (see for example Barbosa & Barbosa 1959, Eckbald 1973, Olsson 1984, Facon et al. 2004, Havel et al. 2014, Poznańska et al. 2015). Gyraulus rossmaessleri appears not only to be a cold-adapted species, as evidenced by its presence beyond the Arctic Circle (Vinarski et al. 2006), and during glacial and late-glacial periods in Western Europe but is also adapted to temporary freshwater environments. To our knowledge, there is no information on the adaptive mechanisms involved and on the extent of its resistance to drought or cold.
Based on our observations in the Alsatian ried ecosystems, we can report the following: (i) the species mainly occupies flood meadows microhabitats, which are best protected from drought and cold; during periods of hydric stress, (ii) individuals may adopt a slower lifestyle, withdraw into their shell which is closed by an epiphragm, (iii) can survive prolonged droughts (several months) and (iv) can become active again as soon as conditions permit (‘quiescent’ sensu Strachan et al. 2015); (v) egg-laying and hatching can follow the first floods a few weeks after the long summer drought; and finally (vi) inundations are likely to be the main dispersal mechanism for the species. These observations need to be validated by improving our knowledge of its micro-distribution and by using appropriate methodological approaches to gain a better understanding of its life cycle and limiting ecological factors.
The lack of key knowledge on the ecology and biology of G. rossmaessleri is a major handicap in implementing appropriate conservation measures (see Lopes-Lima et al. 2021 for an overview), which is categorised as Endangered in France according to IUCN criteria. For example, we cannot estimate the potential impact of surface and/or groundwater flow pollution in the Rhine floodplain by nitrate, which can exceed European standards (Graveline et al. 2014), as are other compounds from pesticide use (Rinaudo et al. 2005). It is also difficult to assess the implications of climate change for the species, particularly regarding an increase in the intensity and duration of droughts and/or a reduction in the duration of floods (Kreins et al. 2015).
The effects of summer mechanical mowing on wet meadows are noted here, as it tends to flatten the soil, eliminating depressions and other micro-reliefs, and increasing the degree of dryness of the soil. Conversely, the closure of open habitats can also lead to the accumulation of organic matter and thus to the filling of depressions. We therefore encourage managers of natural areas to maintain or restore this habitat diversity, which are also home to other highly endangered species such as the branchiopods Lynceus brachyurus Müller, 1776 (Durr & Thiery 2020) and Limnadia lenticularis (Linnaeus, 1761) (Geissert 1961, Thiery et al. 2024), both classified as EN and CR respectively on the French Red List (UICN France & MNHN 2012). To assist conservation, Gyraulus rossmaessleri should be considered as an umbrella species and flagship species, iconic of ried meadows and strictly protected at regional scale.
Finally, this work illustrates also the importance of incorporating data from the fossil record when assessing the conservation status of continental molluscs. Indeed, according to Preece (1998), Quaternary non-marine molluscs are often preserved in different carbonate deposits, making them a valuable tool for temporal conservation purposes. Palaeomalacological data provide a historical perspective to characterise distribution through time, to gain insight into population dynamics and to specify habitat preferences. Comparison of past and modern data reveals a shift towards a highly fragmented distribution, which poses challenges for the study and conservation of the relict taxon Gyraulus rossmaessleri.