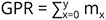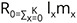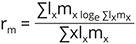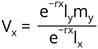INTRODUCTION
The stylommatophoran family Helicarionidae is diverse in South Asia (Bouchet et al. 2017). Helicarionidae include snails with well-developed external shells, semi-slugs, and slugs with reduced internal or external shells. The members of the family are diverse in terms of their life history traits, such as body size, growth, age at first reproduction, fecundity, and longevity (Barrientos 1998, Nylin 2001, Bhosale et al. 2021). Several species in this family cause serious damage to agricultural and horticultural plants (Raut & Ghose 1984, Das et al. 1989). Many other species of terrestrial snails are also recorded as the intermediate hosts of nematode parasites (Hamilton et al. 2020). Many species are vital food sources for birds, reptiles, and mammals (Rosin et al. 2011); hence, they are also important components of the terrestrial food chain (Baur & Baur 1993). Despite being a group with agri-horticultural (Villalobos et al. 1995) and medical importance (Raut & Ghose 1984, D’ávila et al. 2018, Ibrahim et al. 2021) and having the potential for high levels of endemism (Pholyotha et al. 2022), rather little is known of their life history (Cowie 1992, Villalobos et al. 1995, Barrientos 1998, Kuźnik-Kowalska 1999, Silva et al. 2009, Hamilton et al. 2020). There are few studies in the Indian context (Raut et al. 1997, Nandy & Aditya 2022a, 2022b, Nandy et al. 2023).
The land snail Cryptaustenia ovata (H. F. Blanford, 1871) (Stylommatophora: Helicarionidae) is a potent pest of the mulberry plant (Morus sp.) (Das et al. 1989, Saha & Roy 1994a, Avhad et al. 2013). Mitra et al. (2004) give details of its morphology and its distribution in West Bengal. C. ovata is recognised as endemic in the Himalayan region, especially in Darjeeling, West Bengal, India (Ramakrishna & Mitra 2002) as well as Nepal (Kuznetsov & Schileyko 1997, Khanal & Budha 2013). Mossy shadowed rocks and underside of stones are the main habitats of C. ovata at elevations of 1,430 to 1,500 m (Kuznetsov & Schileyko 1997) or at a range between 1,300 and 1,960 m (Khanal & Budha 2013) in Nepal. The snails are also encountered frequently on bamboo fences and tree trunks in the urban habitats of West Bengal (Nandy et al. 2022). There are few other studies on the morphological characteristics, distribution, life histories and habitat preferences of C. ovata, but Saha & Roy (1994b) described egg cannibalism, and found that C. ovata oviposited 34.17 ± 3.34 eggs per clutch with an incubation period of 9 to 26 days.
Studying snail life history features and reproductive strategies helps to understand their adaptability and interaction with the environment and to evaluate population dynamics (Nylin 2001). In addition to growth and development, information on life history traits also involves assessing the adaptive methods of organisms that ensure their fitness and survivability and understanding their strategies of resource allocation to reproduction. All these features are essential in determining age-specific variation in total reproductive value (Sæther et al. 2013), and intraspecific variation in growth and survival (Alonzo & Kindsvater 2008), which can be used as a basis in population ecology to construct a general concept on reproductive fitness of species influenced by natural selection (Baur & Baur 2000). In terrestrial snails, patterns of growth, reproduction and life histories are very diverse. There are few empirical studies on the life history features of terrestrial snails like Helix lucorum (Staikou et al. 1988), Monacha cartusiana (Staikou & Lazaridou-Dimitriadou 1990), Bradybaena fruticum (Staikou et al. 1990), Eobania vermiculata (Mohamed & Ali 2009, 2013), Cochlicella acuta (Mohamed & Ali 2011).
The pestiferous land snails Macrochlamys indica, Lissachatina fulica, and Allopeas gracile (Raut & Ghose 1979a, 1979b, Jahan et al. 2002, Dickens et al. 2018, Nandy & Aditya 2022b) have already been studied to highlight their life history and fecundity features, as an aid to their control. As C. ovata is also a serious pest of commercial plants; this study was designed to observe the growth pattern, oviposition, fecundity schedule, longevity, and other life history traits of this species for the same reasons. As an extension, information obtained on C. ovata will be useful to highlight its pest potential and impact on available crops.
MATERIAL AND METHODS
SNAIL COLLECTION
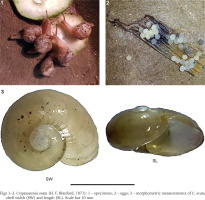
During the monsoon season (July 2016) adult C. ovata were collected from randomly selected unmanaged and partly managed gardens situated in and around Kolkata metropolitan area (22°32'27.96"N, 88°20'16.08"E), West Bengal, India. Adult C. ovata are easily recognised, with a depressed shell, pale white, and slightly descending in front (Fig. 1) (Mitra et al. 2004). The snails were collected by hand from bamboo fences, concrete garden walls, decomposed dead wood of the drumstick tree (Moringa oleifera), leaves and pseudo-stem waste of banana tree, and moist garden soil and kept in plastic containers (Tarson®, India; 100 ml capacity), in which the moisture and humidity were maintained using a wet cotton ball. The collected snails were brought to the laboratory for rearing in a controlled environment.
REARING SNAILS UNDER LABORATORY CONDITIONS
In laboratory conditions, the freshly collected snails were reared in a glass terrarium (30 × 23 × 13 cm), filled with 4 cm thick moist soil at the bottom. Each rearing terrarium had moist and cut leaf sheaths of banana pseudo-stem. The top of the terrarium was covered with a perforated transparent plastic sheet that allowed air circulation while maintaining moisture (Sturm et al. 2006). At least ten such rearing glass terraria were used in this study to maintain all the field-collected snails. The terraria were kept at room temperature (25–27 °C) and water was sprayed inside it to maintain the moist condition and relative humidity. In the rearing terrarium, the snails were fed with slices of cucumber. All the rearing terraria were monitored regularly (Godan 1983, Mohamed & Ali 2009, 2011) and the remaining food, faeces, and dead snails were removed.
EXPERIMENTAL DESIGN
Each of the rearing terraria was monitored daily for the presence of eggs. The snails (P-generation) were found to lay eggs generally under the banana pseudo-stem or on moist soil. They laid eggs in round and gelatinous clutches (Fig. 2). The egg clutches were then transferred in separate plastic containers (100 ml) on the moist soil bed until hatching. An individual cohort was made up of the juveniles that hatched from various clutches on a given day (Mohamed & Ali 2013). Therefore, in each cohort, the number of snails varied. The cohort was maintained in a separate plastic terrarium (16.5 cm in diameter × 10.5 cm in height) with a 2 cm thick moist soil bed and small pieces of banana pseudostem. A total of 11 cohorts (the number of snails in each cohort: n = 8, 36, 10, 29, 9, 30, 60, 30, 7, 10 and 30) were considered for the study. The snails were fed with the slices of cucumber throughout the experiment. The plastic terraria were monitored at regular intervals for maintaining food and moisture, and the occurrence of any dead snails that represented their natural death. The terraria were cleaned at a regular interval of 72 hours.
Measurements of shell length, shell width (Fig. 3), and body weight of at least 7 snails from each cohort were measured weekly to observe the growth pattern. The date of the first oviposition and subsequent date of oviposition were noted for each cohort. The egg clutches were counted and transferred to another container. The last oviposition date was also monitored. The data on fecundity and mortality were also counted to assess the survival, growth, and development of the C. ovata population.
DATA ANALYSIS
Growth was assessed through the change in shell length and body weight as a function of time and tested following the von Bertalanffy growth model with a growth equation for shell length lt = l∞(1 − (e−k(t − t0))), where lt is the shell length (in mm), at time t. l∞, k and t0 are von Bertalanffy parameters (von Bertalanffy 1938, Day & Fleming 1992, Wells & Keesing 2019). Values of k and l∞ of the growth parameters were estimated from the Chapman straight-line equation (y = a + bx) that is constructed from the relationship between lt+1 and lt. The slope b and intercept a represent the functions e(−k) and l∞ × [1 − e(−k)], respectively. The time t is the instantaneous time while t0 is obtained as t0 = t + (1 / k) ln ((l∞ − lt) / (l∞)). The relationship between total length and body weight (W = a × SLb) was employed for the growth equation of body weight as BWt = BW∞(1 − (e−k(t − t0)))b, where BWt is the body weight (in mg) of the snail. The data on the expected and observed values of shell length and body weight were compared using the paired t-test to assess if the growth data fitted the von Bertalanffy growth equation.
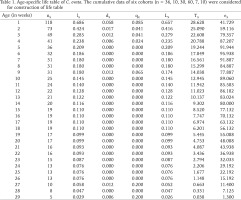
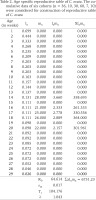
The daily counts of mortality in each cohort were combined to construct the aggregate life table for C. ovata (Krebs 1999). The following life table parameters, such as (nx) = the number of individuals at age x, (lx) = the proportion of the original cohort that survives to age x, (Lx) = lx + lx+1 / 2, (dx) = number of deaths between age x and age x + 1, per capita rate of mortality during the age interval x to x + 1 = (qx) = dx / Lx, and life expectancy at age x = (ex)= sum of Lx / lx were estimated for the life table of snail C. ovata reared in laboratory conditions.
The fecundity schedule was deduced using suitable formulae (Krebs 1999, Smith & Smith 2001) with slight modifications owing to the hermaphroditic nature of the snail. mx represents age-specific fecundity. The gross reproductive rate was calculated by summating the number of eggs produced by the survivors:
To determine the number of offspring produced per individual during life the net reproductive rate was calculated by using the formula:
The cohort generation time Tc = Σxlxmx / R0 and the intrinsic rate of population increase (rm) was obtained as:
The age-specific reproductive values (Vx) were calculated as:
The experiment was statistically analyzed using the XLSTAT software (Addinsoft 2010), following the selection of suitable statistical tests (Krebs 1999, Zar 2009).
RESULTS
ESTIMATION OF GROWTH PATTERN
The shell length (SL), shell width (SW), and body weight (BW) of the snails (n = 259) were measured until their death. Changes in body weight (R2 = 0.994; Fig. 4) and longevity (R2 = 0.926; Fig. 5) of the snails were in increasing function with the shell length and compiled with power equation. The relationship between shell length and shell width followed the linear regression model (R2 = 0.997; Fig. 6). Mean body weight, shell length, and shell width of the 0-day hatched snails were 1.834±0.13 mg, 1.49±0.053 mm, and 1.97±0.057 mm, respectively. The growth measures of six cohorts with the largest number of individuals (n = 36, 10, 29, 30, 60, 30) were considered for the von Bertalanffy growth model. It was observed that the shell length of C. ovata increased until the 17th week and after that, the growth rate gradually reached a stable condition. Comparison of the observed and expected lt values did not show a significant difference (difference = −0.111; p = 0.907; n = 29 paired data), indicating increase in shell length was perfectly fitted with the von Bertalanffy growth equation (Fig. 7). Likewise, the increase in body weight (the body weight of each snail was estimated separately during the study period) was fitted with the von Bertalanffy growth equation, and the observed and expected body weight did not show significant differences (difference = −38.79; p = 0.7; n = 29 paired data; Fig. 8).
SURVIVORSHIP AND LIFE TABLE CHARACTERISTICS
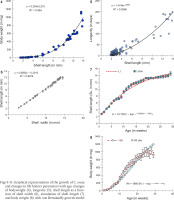
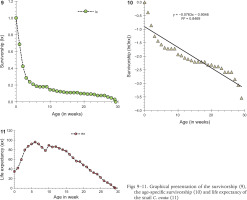
Only six cohorts (n = 36, 10, 30, 60, 7, 10) were considered for the life table construction out of eleven due to early age mortality compared to the rest. Individuals in the cohort had different levels of longevity, with a maximum longevity of 205 days. The survivorship patterns of C. ovata (Figs 9–10) are characterised by high mortality rates at the juvenile stage. The life expectancy of C. ovata is shown in Fig. 11. The mean life expectancy was 58.63 ± 1.99 days. The life expectancy and mortality rate of the snail C. ovata were estimated from the life table data (Table 1), which revealed that the life expectancy of 0-day-old snails was 34.58 days, and the day before death, the life expectancy became 1.3 days. The age-specific life table of C. ovata is shown in Table 1.
ESTIMATION OF FECUNDITY TABLE
The first day of oviposition reflected the date of gaining sexual maturity. Individuals of six cohorts (n = 36, 10, 29, 30, 60, 30) had heterogenous egg production capacities. The onset of the reproductive period occurred between 78 and 111 days when the mean shell length and the corresponding body weight of the snails (n = 21) were 9.09±0.33 mm and 484.97±44.84 mg, respectively, whilst, at last reproduction (day of last oviposition) the mean shell length and body weight of the snails (n = 14) were 10.69±0.23 mm and 744.52±44.3 mg. The oviposition period of C. ovata ranged from 9 and 62 days (in each cohort). Production of eggs per individual increased rapidly with age and then started to fall after the 17th week (we estimated the egg production by dividing the number of eggs laid by alive individuals in the cohort). The net reproductive rate (R0) was 64.614 and the cohort generation time (Tc) was 104.176 days. The intrinsic rate of increase (rm) was estimated at 0.017 and the finite rate of increase (λ) was 1.043. Age-specific reproductive values of C. ovata are shown in Table 2. The data on survivorship, life expectancy and fecundity were utilised to estimate age-specific reproductive values (Vx). The reproductive values were graphically presented as a function of age, which complied with the polynomial regression equation (Fig. 12). Eggs per individual increased gradually with age reaching a peak during the middle of the reproductive period (Fig. 13).
DISCUSSION
The life history traits like body size, longevity, reproductive time, and fecundity reflect the fitness and the pattern of the life history of land snails (Cowie 1984, Jordaens et al. 2007, Dillen et al. 2009). Life-history traits also indicate effective population size ratios, dispersal, and effects of land snail species on the ecosystem (Waples et al. 2013). Apart from being noted as a serious pest of the mulberry plant (Das et al. 1989, Avhad et al. 2013), and other agri-horticultural plants (Raut & Ghose 1984), the information available on the life history features of C. ovata in India was inadequate (Saha & Roy 1994b). Thus, evaluating the life history features of C. ovata provides insight into the adaptive features and consequences at the population level. As studies on helicarionids are scarce, a detailed investigation of the life history features of C. ovata in laboratory conditions was very much imperative and comparison with other members of the same group is expected to help assess its population dynamics (Nagarajan et al. 2021).
C. ovata showed a similar growth pattern as shown by some other stylommatophoran land snails such as Theba pisana (Cowie 1984, Abd-Elhaleim et al. 2022), Limicolaria flammea (Egonmwan 1992), Balea perversa (Baur & Baur 1992), Succinea costaricana (Villalobos et al. 1995), Ovachlamys fulgens (Barrientos 1998), Discus rotundatus (Kuźnik-Kowalska 1999), Habroconus semenlini (Silva et al. 2009), Brevisentis sp. (Hyman et al. 2017), Parmarion martensi (Hamilton et al. 2020), and Allopeas gracile (Nandy & Aditya 2022b). The growth rate was maximum at the initial stage (first 9th-10th week) of the life cycle and continued to increase till the 17th week and then gradually reached a stable condition. As observed in helicarionid snail O. fulgens (Barrientos 1998), the growth pattern of C. ovata is an indeterminate type where the growth continues even after attaining sexual maturity (Carvalho et al. 2008).
The maximum survival time of the snail C. ovata in laboratory culture was about 29 weeks (n = 5). In contrast, the other stylommatophoran snails, such as O. fulgens (Barrientos 1998), S. costaricana (Villalobos et al. 1995), P. martensi (Hamilton et al. 2020) have a life span of about 36, 44, 48 weeks, respectively. While the snail Monacha cartusiana (Al-Doori et al. 2023) and A. gracile (Nandy & Aditya 2022b) reared in cohorts of multiple individuals showed an even shorter life span of about 21 and 27 weeks respectively, other stylommatophorans such as Theba pisana (Cowie 1984), Eobania vermiculata (Mohamed & Ali 2009), Helicodonta obvoluta (Maltz 2003), Discus rotundatus (Kuźnik-Kowalska 1999) have a comparatively long lifespan of 104, 131 156, 182 weeks, respectively; these are iteroparous species. In this study, C. ovata exhibited high mortality in the early and late middle of life. A high mortality level in early life is probably because of increasing intraspecific competition as the hatchlings appear to increase population density as observed for Helix lucorum (Staikou et al. 1988).
Many terrestrial pulmonates generally gain rapid sexual maturity that often lasts for a short period (Baur 1989, Saha & Roy 1994b), while few are long-lived (Cameron 2016). Several studies showed that the earliest oviposition began on about 20, 42, and 93 days after eclosion in the case of snails A. gracile (Nandy & Aditya 2022b), O. fulgens (Barrientos 1998), B. perversa (Baur & Baur 1992), respectively. There are exceptions such as Discus rotundatus (Kuźnik-Kowalska 1999), Helicodonta obvoluta (Maltz 2003), and Hawaiian Achatinella (Cowie 1992) that require about 1–2 and 4–7 years, respectively for sexual maturation. In C. ovata, the onset of reproductive event was observed on the 11th week when the mean shell length was 9.09±0.33 mm. In another study, C. ovata showed a reproductive phase of 9 weeks, and during this period the snail oviposits 34.17±3.34 eggs per clutch with an incubation period of 9 to 26 days (Saha & Roy 1994b).
In multiple individual cohort cultures, C. ovata oviposited in small clutches within a short reproductive period at a very high frequency every 8.81±1.18 days, which is similar to O. fulgens, Zonitoides nitidus that lays eggs in clutches at an interval of every 9–13 days (Didier & Rondelaud 1987, Barrientos 1998). This oviposition strategy of C. ovata is an adaptive life history trait modality of snails as a pest, which may be associated with rapid population dispersal and establishment in a new habitat, as predicted for the slug Mariaella dussumieri (Barman et al. 2022) and other snails (Raut & Ghose 1984). Since, the humidity, temperature, soil moisture, and availability of food determine the survivability and colonisation potency of the species in a new habitat, the observations carried out in laboratory conditions may deviate from what happens in the natural environment, where the availability of resources and habitat structure may vary. Therefore, assessment of life history traits under varying degrees of environmental factors is further required to validate the influence of biotic and abiotic factors on the life history features of C. ovata. Nevertheless, the present investigation on C. ovata substantiates its early reproductive maturity and rapid growth rate (Bengtsson & Baur 1993). This study of the mortality, fecundity, and growth measurement of the pest snail C. ovata provides important data for managing their population dynamics that enabled prediction about the extent of infestation and population abundance of the snails.