Darwin (1877) described a sexual polymorphism as the co-occurrence of sexual morphs within the same population. In gastropods we are dealing with a phally polymorphism which is a type of genital polymorphism in hermaphroditic organisms (Doums et al. 1998). In Vertigo snails, in one population, we may find three different sexual morphs: euphallics which have a fully developed and functional penis, hemiphallics which have vestigial and non-functional penis, as well as aphallics which totally lack penis. All morphs are able to self-fertilize, but only euphallics can transfer sperm to a mating partner during copulation (Pokryszko 1987, Doums et al. 1998).
The aphally in the Vertiginidae was described by Watson (1923) and Steenberg (1925) in the first half of the 20th century. More than sixty years later, Pokryszko (1987) extensively studied this phenomenon in Vertigo snails. Her studies focused mostly on animals collected from Poland, but she also dissected some individuals from other countries such as Great Britain, Norway and Sweden. Shortly afterwards, her monograph on the European Vertiginidae (Pokryszko 1990) gave a detailed description of diversity in copulatory organs in representatives of this family. A recent study on phally polymorphism in some European Vertigo species was published by Książkiewicz et al. (2023).
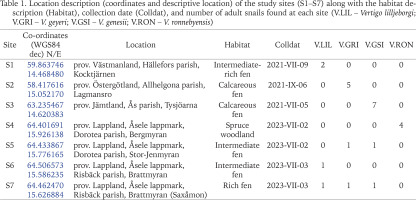
Here, we present new data on the phally polymorphism in snails collected in Sweden. The data concern four Vertigo species: Vertigo lilljeborgi, V. geyeri, V. genesii, and V. ronnebyensis. Snails for the study were acquired from 7 sites in total: two in central Sweden (1–2) and five in the northern part of the country (3–7) (Table 1).
Table 1
Location description (coordinates and descriptive location) of the study sites (S1–S7) along with the habitat description (Habitat), collection date (Colldat), and number of adult snails found at each site (V.LIL – Vertigo lilljeborgi; V.GRI – V. geyeri; V.GSI – V. genesii; V.RON – V. ronnebyensis)
Snails were collected using a combination of two methods: 1) Shaking decaying plant material, grass and mosses in a metal bowl and collecting the specimens by hand from the bottom of the bowl directly in the field; 2) Sieving decaying plant material, grass and mosses in a sieve with mesh size of 10 mm. The material passing through the sieve was brought back to the laboratory and the snails picked out by hand under a magnifying glass. Only specimens with a complete shell (adults) were included in the study. Collected individuals were preserved in 96% alcohol.
Snails were identified to species according to Pokryszko (1990) and von Proschwitz et al. (2023). All individuals tested for the phally polymorphism were adults i.e. had a fully developed shell with the completed apertural barriers. The shell of each of the snail was measured with the OLYMPUS SZX16 stereo microscope with OLYMPUS DP74 digital camera and software: cellSens Dimension and Helicon Focus 7. Measurements included shell height (referred in tables as H1), body whorl height (H2), aperture height (H3), aperture width (W1), shell width (W2). All measurements were taken after Pokryszko (1990). After taking the measurements, snails were dissected and the sexual morph was determined also according to Pokryszko (1990).
Aphallics were found in all studied species, euphallics were detected in three of them (V. lilljeborgi, V. geyeri, V. genesii), and hemiphallics in two of them (V. geyeri, V. genesii) (Tables 2–5). Pokryszko (1987, 1990) detected just two sexual morphs: euphallics and aphallics in all studied species. The individuals she dissected were found in Poland (V. ronnebyensis – 5 individuals), England (V. genesii – 6 individuals and V. geyeri – 7 individuals), as well as Norway, Sweden, Great Britain, and USSR (V. lilljeborgi – 61 individuals). Although the material we present does not allow comparative analysis of the morph frequencies, we report for the first time hemiphallics in V. geyeri and V. genesii populations.
Table 2
Phally morphs and shell dimensions [mm] in adults of V. lilljeborgi: H1 – shell height; H2 – body whorl height; H3 – aperture height; W1 – shell width; W2 – aperture width; A – aphallics; E – euphallics
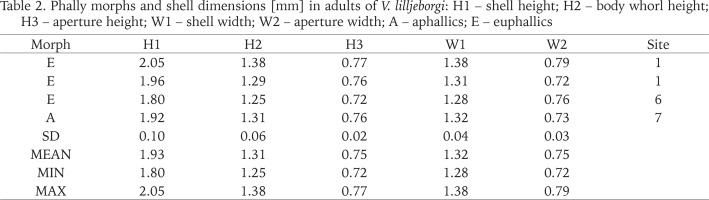
Table 3
Phally morphs and shell dimensions [mm] in adults of V. geyeri: H1 – shell height; H2 – body whorl height; H3 – aperture height; W1 – shell width; W2 – aperture width; E – euphallics; A – aphallics; H – hemiphallics
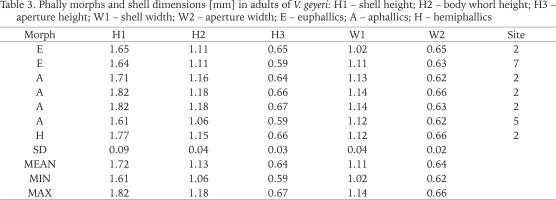
Table 4
Phally morphs and shell dimensions [mm] in adults of V. genesii: H1 – shell height; H2 – body whorl height; H3 – aperture height; W1 – shell width; W2 – aperture width; E – euphallics; A – aphallics; H – hemiphallics
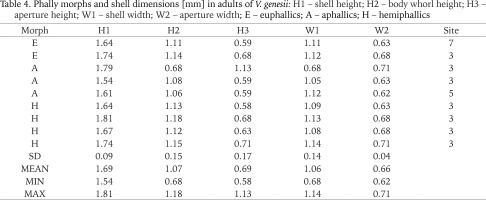
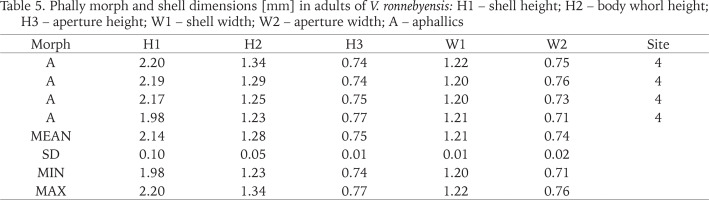
Table 5
Phally morph and shell dimensions [mm] in adults of V. ronnebyensis: H1 – shell height; H2 – body whorl height; H3 – aperture height; W1 – shell width; W2 – aperture width; A – aphallics
Comparing shell descriptions provided by Pokryszko (1990), descriptions provided by von Proschwitz et al. (2023) and measurements taken by us, we also noticed the Swedish snails tend to be smaller than those studied by Pokryszko (1990). We recorded following shell dimensions for V. lilljeborgi in Sweden (minimum and maximum values) H1: 1.80–2.05 mm; W1: 1.28–1.38 mm (Table 2). To compare, Pokryszko (1990) provided following shell dimensions for the species H1: 2.00–2.35 mm; W1: 1.15–1.33 mm, while von Proschwitz et al. (2023) H1: 1.70–2.40 mm; W1: 1.10–1.40 mm.
In the case of the Swedish V. geyeri the shell dimensions were as follows H1: 1.61–1.82 mm; W1: 1.02–1.14 mm (Table 3). Dimensions provided by Pokryszko (1990) for the species were: H1: 1.63–1.93 mm; W1: 1.05–1.20 mm; dimensions recorded by von Proschwitz et al. (2023): H1: 1.60–2.00 mm; W1: 1.00–1.20 mm.
Shell dimensions of V. genesii in Swedish individuals were as follows H1: 1.54–1.81 mm; W1: 0.68–1.14 mm (Table 4). Dimensions provided by Pokryszko (1990) for the vertiginid were: H1: 1.63–2.00 mm; W1: 1.03–1.20 mm and by von Proschwitz et al. (2023) H1: 1.60–2.10 mm; W1: 1.00–1.20 mm.
In the case of V. ronnebyensis we recorded following minimum and maximum dimensions H1: 1.98–2.20 mm; W1: 1.20–1.22 mm (Table 5). Pokryszko (1990) provided shell dimensions as follow H1: 2.00–2.32 mm; W1: 1.15–1.33 mm; von Proschwitz et al. (2023): H1: 2.00–2.40 mm; W1: 1.10–1.40 mm.
In conclusion, this short study revealed that our knowledge on the phally polymorphism is just the tip of an iceberg. Dissections of a small number of snails revealed new data on the hemiphally on the two Vertigo species: V. genesii and V. geyeri. Previous studies suggested that the hemiphallics may be “enfeebled” euphallics, and occur in high numbers in somehow disturbed environments (Książkiewicz et al. 2023). Facing increased anthropogenic pressure and the climate crisis we may therefore suspect an increased share of hemiphallics in populations. Since this sexual morph is unable to transfer sperm during the copulation, such disturbed balance of sexual morphs may have profound effect on the genetic diversity of the species populations. Therefore, we emphasize the need for more extended research on the phally polymorphism to understand better its background and consequences for Vertigo species conservation.

