INTRODUCTION
The study of biological invasions is essential not only to assess their environmental impacts and economic costs but also to understand their biogeographic processes, such as long-distance dispersal, rapid adaptation, and range expansion (Viard et al. 2016). Thus, it is crucial to acquire the most up-to-date information regarding their invasiveness beyond their native range (Ulman et al. 2017, Paganelli et al. 2021).
The large unionid Chinese Pond Mussel Sinanodonta woodiana (Lea, 1834) (Mollusca: Bivalvia) is one of the most invasive alien macro-invertebrates worldwide (Colomba et al. 2013, Paganelli et al. 2021). Originally from Yangtze basin and southern China (Kondakov et al. 2018, Konečný et al. 2018, Douda et al. 2024). This freshwater bivalve has, during the last 50 years, expanded its range at an increasing pace throughout Europe (Kraszewski 2007 and literature therein, Konečný et al. 2018, Spyra et al. 2016, Huber & Geist 2019, Urbańska et al. 2021), Asia (Kondakov et al. 2020a, 2020b, Bolotov et al. 2022), and more recently, Africa (Mabrouki & Taybi 2022, Bensaâd-Bendjedid et al. 2023). It is commonly assumed that the release of host fish bearing the mussel’s larval glochidia during fish stocking is often the principal pathway of its dispersal into new aquatic habitats (Sárkány-Kiss et al. 2000, Douda et al. 2012, Spyra et al. 2016, Urbańska et al. 2021, Dobler et al. 2022), in addition to releases from pet shop trade which seems to be a problem in parts of Europe (Benedict et al. 2024).
However, the broad tolerance of S. woodiana to environmental conditions (variety of substrates, extreme climatic conditions, hypoxia and eutrophication) and its high resistance to anthropogenic impacts (pollution, habitat degradation and fragmentation) associated with remarkable morphological plasticity, a high degree of fecundity, and an indeterminate growth pattern, make S. woodiana a successful invader (Dudgeon & Morton 1983, Kraszewski 2007, Guarneri et al. 2014, Sousa et al. 2014, Labecka & Domagala 2018, Labecka & Czarnoleski 2021, Benedict & Geist 2021, Urbańska et al. 2021, Dobler et al. 2022, Geist et al. 2023). Once the species has been introduced, it can outcompete native mussels by its greater reproductive output compared to native mussel species (Huber & Geist 2019, Labecka & Czarnoleski 2021). As a consequence, it is assumed that its spread outside its native range has dramatic effects on local fauna, resulting in a decline or total replacement of indigenous anodontine species (Najberek et al. 2011, Benkő-Kiss et al. 2013, Cilenti et al. 2019, Karaouzas et al. 2022, Geist et al. 2023).
In Algeria, to date, the only documented records of the Chinese Pond Mussel have been reported by Bensaâd-Bendjedid et al. (2023) and Belkacem et al. (2023) in the UNESCO Biosphere Reserve Oubeira Lake and Beni Haroun Dam reservoir, respectively. These authors agreed that, at both sites, S. woodiana was most likely involuntarily introduced along with Asian carp fingerlings imported from Central Europe. Furthermore, due to the species’ high level of invasiveness and the broad interconnection of hydrographic systems, Bensaâd-Bendjedid et al. (2023) and Douda et al. (2024) predicted new reports of S. woodiana presence at a regional scale in North Africa.
Babar Dam (District of Khenchela) located in the eastern part of the Saharan Atlas, is one of the most important hydraulic structures exploited for agriculture and tourism development in Northeast Algeria (Gaagai 2017). Built in a semi-arid region at an altitude of more than 1000 m, the dam is primarily used for water supply for domestic purposes as well as to irrigate thousands of hectares of farmland (Tebbi et al. 2018, Gaagai et al. 2020a). In the context of a broad national strategy for aquaculture development (1985–2009), Babar Dam reservoir was subject, along with more than twenty other sites, to the release of approximately 6 million silver (Hypophthalmichthys molitrix) and bighead carp (Aristichthys nobilis) juveniles imported from Hungary (Meddour et al. 2010, Seridi 2011, Kara 2012). Nevertheless, by the 2000s, several scientists began to warn against the adverse environmental consequences of the introductions of exotic Cyprinidae, as the latter have been found to be vectors for the spread of numerous pests and pathogens across Algerian aquatic ecosystems (Meddour et al. 2005, 2010, Kara 2012, Khelifi et al. 2018, Bensaâd-Bendjedid et al. 2023). This contribution is one further piece of evidence for these findings. In this paper, we report Babar Dam reservoir as a new site for the occurrence of the invasive Chinese Pond Mussel and the southernmost record of the species in the country’s inland waters. Additionally, we present a preliminary characterisation of the species population status at the study site and discuss the possible introduction pathway as well as the potential distribution of S. woodiana throughout the entire Algerian territory.
MATERIALS AND METHODS
STUDY AREA
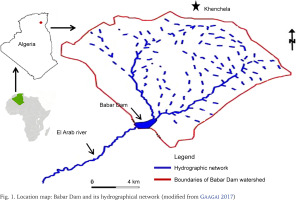
Babar Dam (Fig. 1) (altitude: 1,109 m; capacity: 38,000,000 m3; watershed surface area: 571 km2) is situated in Northeast Algeria (35°10'20.4"N, 7°01'46.1"E), between the Aurès and Nemencha mountains, at the extreme eastern limits of the Saharan Atlas (Gaagai 2017, Tebbi et al. 2018, Achour et al. 2019). This hydraulic structure was constructed in 1989, a few kilometers from the city of Khenchela to retain the waters of the El Arab River, which is included in the catchment area of Chott Melghir (Ramsar site no. 1296), a large complex of seasonal salt lakes and freshwater pools representative of arid and hyper-arid Saharan environments (Tebbi et al. 2018, Koussa & Bouziane 2019, Gaagai et al. 2020a). Babar reservoir has an arid/semi-arid climate and is characterised by low-turbidity water, which ranges in temperature from 7 to 32 °C; the pH is slightly alkaline, near neutrality (Achour et al. 2019, Gaagai et al. 2020b).
BIOLOGICAL SAMPLING AND DATA PROCESSING
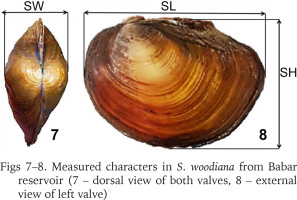
S. woodiana was discovered in Babar Dam reservoir (Fig. 2) on May 2023, during a field survey of the wildlife and avifauna in the semi-arid wetlands of eastern Algeria. The mussels were easily found along the reservoir’s banks and manually gathered at depths of 0 to 30 cm (Figs 3–6). Only live specimens were photographed and processed. Bivalves were measured (mm) along their maximum shell length (SL), width (SW), and height (SH) (Figs 7–8) with a digital calliper to the nearest 0.1 mm. To obtain individual total body mass (BW), mussels were wiped with absorbent paper and then accurately weighted (g) using a digital scale with a precision of 0.01 g. The species identification was based on a combination of diagnostic criteria, including the external shell colour (ranging from light brown to dark brown and black), internal shell colour (pinkish-white), and form (wide, rounded, swollen, thin, and fragile), the sculpture of the umbo (4–8 prominent, spaced, subconcentric or slightly transverse ripples) and hinge features (absence of teeth) (Girardi & Ledoux 1989, Kiss 1995, Popa et al. 2007, Adam 2010, Zieritz et al. 2012, Colomba et al. 2013).
Figs 3–6
Sinanodonta woodiana from Babar reservoir: 3 – live mussels; 4 – empty shell of S. woodiana; 5 – external and internal views of the shell (SL = 147.3 mm); 6 – details of umbonal sculptures (SW = 66.1 mm)

Figs 7–8
Measured characters in S. woodiana from Babar reservoir (7 – dorsal view of both valves, 8 – external view of left valve)
The population structure of S. woodiana from Babar Dam was determined from total shell length (SL) according to the method of Afanasjev et al. (2001) and Spyra et al. (2012), through the classification of mussels into four size groups: young (< 50 mm), small (50 ≤ SL ≤ 100 mm), medium (100 < SL ≤ 150 mm) and large (SL > 150 mm). The age of each sampled individual was estimated by counting the surface annual growth rings. The statistical analysis was performed using Excel 2010 (Microsoft Office) software.
Additionally, data mining research on social networks (iNaturalist and Facebook) combined with a literature survey allowed us to broaden the scope of our study and present S. woodiana’s potential introduction pathway and distribution in Algeria. We collected as much data as possible on recent confirmed records as well as the alleged finds of the species reported by amateur naturalists and fishermen. Once analysed, a scenario for new range-extending records of the Chinese Pond Mussel in Algerian inland water has been elaborated.
RESULTS AND DISCUSSION
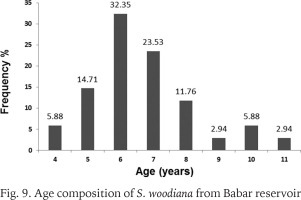
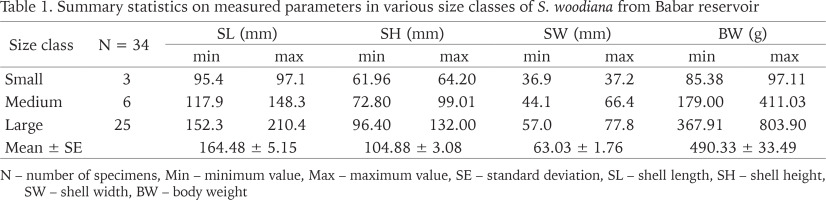
Within the framework of this study, the total sample included 34 live Chinese Pond Mussel specimens. Basic statistics for shell dimensions and body weights of S. woodiana from Babar Dam are detailed by size classes in Table 1. Total shell length and body mass ranged between 95.4 and 210.4 mm and from 85.38 to 803.9 g, respectively. Comparing our data with the previous records of this species in Africa, it appears that the maximum shell length obtained for the mussels in the study site exceeded the sizes reported from Algeria: Oubeira Lake (183.55 mm, Bensaâd-Bendjedid et al. 2023) and Beni Haroun Dam (167 mm, Belkacem et al. 2023) as well as in Morocco: Al Kansra Dam (170 mm, Mabrouki & Taybi 2022). However, it remained lower than that observed in some invaded European water bodies where S. woodiana shells have reached 250–270 mm (Kraszewski & Zdanowski 2007, Lajtner & Crnčan 2011, Dobler et al. 2022). Several authors attributed intraspecific differences in the growth of the Chinese Pond Mussel populations to environmental factors, especially water temperature (Soroka 2000, Kraszewski 2006, Spyra et al. 2016). For instance, in Poland, according to Kraszewski & Zdanowski (2007), the species reaches maximum shell length (above 200 mm) in anthropogenically heated lakes rather than moderately warm waters. Furthermore, Kiss (1995) noted that during hot summer periods, the growth rate of S. woodiana may be twice as fast as during the cold winter months. In Babar Dam, the upper water temperature limit exceeds 30 °C with an annual mean of about 17 °C (Gaagai et al. 2020b), which provides the optimum thermal conditions described for the species (Kraszewski & Zdanowski 2001, Demayo et al. 2012), and might explain its establishment and remarkable development. Indeed, except for the younger mussels (5 cm), all size classes of S. woodiana were present in Babar Reservoir. Large specimens constituted the highest proportion, while the medium-sized mussel class was second in terms of abundance (Table 1). On the other hand, the age structure of the sampled bivalves was formed into eight groups ranging from 4 to 11 years old (Fig. 9). Six- and seven-year-old mussels dominated the analysed population. These findings suggest that at the study site, S. woodiana has successfully established a stable and naturalized population capable of sustaining itself. According to several authors, the presence of individuals of various sizes and ages indicates the adaptation of the Chinese Pond Mussels to the invaded ecosystem conditions and their ability to grow and reproduce there (Soroka 2000, Cappelletti et al. 2009, Urbańska et al. 2012, Kamburska et al. 2013).
Table 1
Summary statistics on measured parameters in various size classes of S. woodiana from Babar reservoir
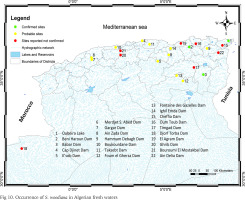
Concerning the potential introduction pathway and distribution of S. woodiana in Algeria, by referring to Bensaâd-Bendjedid et al. (2023) and Belkacem et al. (2023), the Chinese Pond Mussel was most likely unintentionally introduced as glochidia (obligatory parasitic larval stage) hosted by farmed Asian carp species (Hypophthalmichthys molitrix, Aristichthys nobilis) imported from Hungary and widely released in Algerian inland waters within the framework of a broad national program for aquaculture development established between 1985 and 2009. This assumption appears well-founded and justified, even though verification by genetic methods is necessary. In fact, Hungary has been proven to be the starting spot, along with Romania, for the spread of S. woodiana across the European continent (Watters 1997, Kraszewski & Zdanowski 2001, Urbańska et al. 2012, Konečný et al. 2018, Douda et al. 2024, Mehler et al. 2024). In addition, to date, the three locations where the species was confirmed, namely Oubeira Lake (Bensaâd-Bendjedid et al. 2023), Beni Haroun (Belkacem et al. 2023), and Babar (present study) Dam reservoirs, were all subjects of intensive introductions of exotic carp fry produced in Hungarian hatcheries (Algerian Ministry of Fisheries and Fish Resources in Seridi 2011). Furthermore, S. woodiana was also anecdotally reported by fishers and amateur naturalists in numerous water bodies across the country. Even though these alleged occurrences should be checked and the species formally identified, it is significant that the common thread between these sites is that they have hosted at least one stocking event during the period mentioned above (Table 2). Based on this, we hypothesize that S. woodiana is probably prevalent in most water bodies where non-autochthonous fishes imported from Hungary were released. Under this scenario, we identified 22 locations as recipient habitats for the species. Figure 10 summarises the current and alleged localities of the Chinese Pond Mussel in Algerian water bodies. If our assumption is correct, we estimate that, given the significant invasive potential of the Chinese pond mussel and the broad interconnection of hydrographic systems (Fig. 10) its distribution in Algeria is underestimated and probably wider than our study data suggest.
Table 2
History of fish stocking events related to the importation of fingerlings from Hungarian hatcheries (Algerian Ministry of Fisheries and Fish resources in Seridi 2011)
CONCLUSION
In Algeria, ecological and biological studies of S. woodiana have just started; the species was probably introduced a long time ago, although it was only first reported in 2023. Investigating S. woodiana’s distribution, population dynamics, reproduction potential, and habitat preferences as quickly as possible is crucial and highly advised since these are the key that will determine the level of the mussel’s impact on Algerian-invaded ecosystems and serve as a starting point for establishing a management strategy to deal with its presence and prevent further spreading.