Bythinella Moquin-Tandon, 1856, with its type species Bulimus viridis Poiret, 1801, belongs to the family Bythinellidae Locard, 1893. For a long time, it was classified within the Amnicolidae Tryon, 1863, but both morphology (Szarowska 2006) and molecular data (Wilke et al. 2001, 2013) confirmed the distinctness of the Bythinellidae. There are about 250 nominal species of Bythinella (WoRMS 2024). However, neither morphology (Giusti & Pezzoli 1977, Falniowski 1987, 2018, Mazan 2000, Haase et al. 2007, Jaszczyńska in press), nor molecular loci studied so far (Bichain et al. 2007a, b, Benke et al. 2009, 2011, Falniowski et al. 2009a, Wilke et al. 2010, Falniowski & Szarowska 2011, Szarowska et al. 2016, Jaszczyńska in press) may be used to separate any natural taxon of the genus level within the Bythinella.
Sitnikova et al. (1992) described from Ukraine a genus Terrestribythinella Sitnikova, Starobogatov et Anistratenko, 1992, with its type species T. baidashnikovi Sitnikova, Starobogatov et Anistratenko, 1992. The other species: T. carpathica Sitnikova, Starobogatov et Anistratenko, 1992 was described and new family Terrestribythinellidae Sitnikova, Starobogatov et Anistratenko, 1992 was created in the same publication (Sitnikova et al. 1992). Later, the third species of Terrestribythinella: T. amphibiotica Anistratenko, 1995 was described (Anistratenko 1995).
According to Sitnikova et al. (1992) there is no tubular penial gland in the male reproductive organs of Terrestribythinella. There should be said that in such case the presence of a big flagellum would be enigmatic, and most probably the tubular gland was small and thus overlooked. In the female reproductive organs, according to Sitnikova et al. (1992), there was a diauly [meaning the existence of a distinct sperm duct (Davis 1967, Hershler & Ponder 1998) – resulting in two separate female genital openings]. The diauly is characteristic for the Amnicolidae, but not for the Bythinellidae, since in the latter very broad folds forming the ventral channel mimic a spermathecal duct (Szarowska 2006). According to Sitnikova et al. (1992), there is also a somewhat curious bursa with duct, and no receptaculum seminis. In fact, both bursa and receptaculum are extremely variable in Bythinella (e.g. Giusti & Pezzoli 1977, Falniowski 1987, 2018, Mazan 2000), the receptaculum may be as small as to be easily overlooked. Considering the poor fidelity of the reproduction of the drawings of the shells and reproductive organs of Falniowskia neglectissima (Falniowski et Šteffek, 1989), the type species of Falniowskia Bernasconi, 1990, redrawn from the original description (Falniowski & Šteffek 1989), the drawings and descriptions of Sitnikova et al. (1992) are thus even less convincing. Thus, these morphological characters alone may not constitute the base of systematics in the case of Bythinella and other truncatelloidean gastropods (Falniowski 2018). Molecular data are therefore necessary for clearer taxonomic conclusions.
For Terrestribythinella we performed molecular data analysis following standard methods (Falniowski et al. 2023). The cytochrome c oxidase subunit I (COI) – a marker commonly used in animals’ barcoding – definitely placed Terrestribythinella within the Bythinella, as two species (mOTU A and B; Fig. 1), forming a sister clade with B. viseuiana Falniowski, Szarowska et Sirbu, 2009 (Falniowski et al. 2009b; mOTU C), and close to the other Bythinella species (mOTUs D–G) from Romania, whose localities were not far from the Ukraine. Such close relationships rather definitely deny the possibility of such substantial anatomical differences as the ones listed above, described by Sitnikova et al. (1992). Terrestribythinella clearly belongs to the genus Bythinella, and it cannot be accepted as a genus without creating a paraphyletic status of Bythinella. Thus, the only solution is to consider Terrestribythinella a junior subjective synonym of Bythinella Moquin-Tandon, 1856. Phylogenetic analyses using all available Bythinella sequences also support this thesis (Benke et al. 2011, Jaszczyńska in press).
Fig. 1
Maximum likelihood tree based on the COI for Terrestribythinella and related taxa, bootstrap supports (>60%) and Bayesian posterior probabilities are given. The HKY+I+G model were used for ML and BI analysis. Results of species delimitation are also shown. The GenBank numbers are given with the taxon, the sequences after Wilke et al. 2000, 2001, Szarowska & Wilke 2004, Bichain et al. 2007a, Falniowski et al. 2009a, Benke et al. 2011, Anistratenko et al. 2021 (unpublished) and present paper
Another genus reported in the Bythinellidae in WoRMS (2024) is Strandzhia Georgiev et Glöer, 2013, with its type species S. bythinellopenia Georgiev et Glöer, 2013. Its placement within the Bythinellidae remains enigmatic, since it was described as a member of the Hydrobiidae (Georgiev & Glöer 2013). Our sequences of COI deposited in GenBank (PP752094-PP752096), as well as the one published by Delicado et al. (2024: OP096318) undoubtedly classify S. bythinellopenia as belonging to the genus Grossunana Radoman, 1983, since in our tree Strandzhia bythinellopenia clusters within the mOTU A (Fig. 2), together with Grossuana derventica Georgiev et Glöer, 2013, G. falniowskii Georgiev, Glöer, Dedov et Irikov, 2015, and G. thracica Glöer et Georgiev 2009. The p-distance within this group does not exceed 0.001, and between this group (mOTU A) and other species of Grossuana (Falniowski et al. 2016) varied from 0.013 to 0.064 (mOTUs B-I). The distance 0.001 is much below the threshold value generally accepted between congeneric species (e.g. Prié & Bichain 2009, Prié & Cucherat 2021). Also, 18S sequence (GenBank PP752097-PP752099) confirmed the assignment of Strandzhia bythinellopenia to the genus Grossuana (Fig. 3). Our dissection of paratypes of this species showed the simple penis typical of Grossuana. This, coupled with careful examination of the photograph published by Georgiev & Glöer (2013) clearly show that the region where the simple penis is bent typically for Grossuana, was erroneously interpreted as the base of bi-armed penis, and the base was misrecognised as the tip of the second arm. To conclude, Strandzhia Georgiev et Glöer, 2013 becomes a junior objective synonym of Grossuana Radoman, 1983.
Fig. 2
Maximum likelihood tree based on the COI for Strandzhia and related taxa, bootstrap supports (>60%) and Bayesian posterior probabilities are given. The HKY+G model were used for ML and BI analysis. Results of species delimitation are also shown. The GenBank numbers are given with the taxon name, the sequences after Szarowska et al. 2007, Falniowski et al. 2012, Falniowski et al. 2016, Hofman et al. 2021, Delicado et al. 2024 and present paper
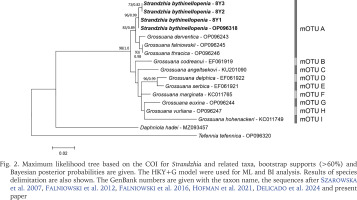
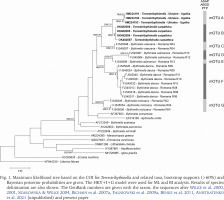
Fig. 3
Maximum likelihood tree based on the 18S for Strandzhia and related taxa, bootstrap supports (>60%) and Bayesian posterior probabilities are given. The HKY+G model were used for ML and BI analysis. The GenBank numbers are given with the taxon name, the sequences after Szarowska et al. 2007, Çağlan et al. 2012, Falniowski et al. 2012, Falniowski & Szarowska 2013, Delicado et al. 2024 and present paper


